Taskforces
INTERDEM Task Forces (TFs) are of key importance to the growth and sustainability of psychosocial dementia care across Europe. They are developed dynamically to reflect current gaps and questions for improving research and associated practice in the support of people with dementia, families and paid staff. Cross cutting activity for example overlapping work with methodologies, technologies or a particular topic of interest is encouraged.
Since 2023 our strategies now include TFs as a standing item at monthly Board meetings; TF Leads /Co-leads invited to Board meetings to share their work; TF workshops at our annual INTERDEM meeting; TF webinars; and structured annual updates of information, aims, achievements of work disseminated on this website (below).
Growth has been encouraging with a ceiling of nine TFs, huge interest from a growing membership including academy- early career researchers, where many have joined and are contributing to several TFs.
INTERDEM Task Forces Guidelines
Current Taskforces are:
Young-onset Dementia
Leads: Dr. Christian Bakker, Radboudumc Alzheimer Center, Nijmegen, The Netherlands Christian.Bakker@radboudumc.nl (lead); Dr. Esther Gerritzen, Institute of Mental Health, University of Nottingham, Nottingham, United Kingdom esther.gerritzen@nottingham.ac.uk (co-lead).
Contact: Esther Gerritzen esther.gerritzen@nottingham.ac.uk
Established: 2023 Annual INTERDEM meeting at Alzheimer Europe, Helsinki, Finland.
Updated: October 2025
Background
In the past decades psychosocial research in young-onset dementia has focused on themes such as the exploration of lived experiences of individuals with dementia onset prior to the age of 65 and their families, the course of care and support needs, and the development of psychosocial support. Previous research emphasizes the unique challenges faced by people living with young-onset dementia and their families and underlines the multifaceted nature of its psychosocial impact. For instance, given the low prevalence of young-onset dementia1, the specific care needs of this particular group are easily overlooked in dementia healthcare2. People living with young-onset dementia often experience stigma, and face challenges in employment, social contexts, and in maintaining a sense of identity and wellbeing3-5. Furthermore, young-onset dementia can be associated with a heightened caregiver burden and strains on interpersonal relationships and family dynamics6, 7.
People with young-onset dementia and their families experience a lack of fit between their needs and preferences, and existing dementia care and support services (including psychosocial support)2, 8. This may not be surprising as these services have been developed with the needs of those living with dementia in old age in mind. There have been few research projects aimed at the development of care and support in young-onset dementia. This includes research regarding interventions aimed at supporting people living with young-onset dementia to keep engaged and socially connected9, 10 and support programs for caregivers11, demonstrating promise in enhancing overall well-being of those affected and their families. However, further research is essential to expand supportive strategies in young-onset dementia with a more personalized, integrative, and family oriented approach.
Overall aim
Since there are differences in dementia care systems across Europe, this INTERDEM Taskforce aims to improve care and psychosocial support for people with young-onset dementia, and to establish how this can best be integrated into current social and healthcare systems.
Activities for 2025 – 2027
- Continue the work in the different special interest groups (SIG) that have been set up within the Taskforce. The SIG are centred around the following themes:
- Dementia in the workplace (SIG leads: Louise Ritchie and Bo Smeets)
The work in this SIG focusses the exchange of crosscountry knowledge on work and dementia. Currently a position paper is in preparation: Work and Dementia: Developing pan-European consensus on support for continued employment post diagnosis of dementia.
-
- Care pathways in young-onset dementia (SIG leads: Janne Papma and Christian Bakker)
The work in this SIG has focussed on setting-up cross-national projects on inventorying (online) resources for young carers (Lead: Patricia Masterson) and a Delphi-study for establishing recommendations for age-appropriate care pathways in YOD (Lead: Christian Bakker). Members are also preparing for a position paper on care pathways in YOD.
-
- Online support (SIG leads: Catherine Talbot and Aysegul Kafadar)
The work in this SIG has focussed on writing a position paper on this topic, expected to be published end 2025. Furthermore, opportunities are explored for a cross-country online survey exploring attitudes and availability of online support (sampling professionals, experts, and people with lived experience)
-
- Capacity building (SIG leads: Laura Cole & Stevie Hendriks)
Aim of this SIG is to support early career YOD researchers in their career development. Activities will focus on:
- Provide international webinars, including INTERDEM Spotlight
- Collaborate with YOD Knowledge Center & YOD-INCLUDED (the Netherlands) and Young Dementia Network (UK)
- Opportunities for YOD taskforce to support INTERDEM Academy Travel Fellowships
- Establish collaborations for meaningful PPIE
- Facilitate peer support for early career YOD researchers.
- Identify and prioritize other themes that should be addressed by the taskforce and set up SIG’s accordingly. This will be done through literature search and consulting people living with young-onset dementia, potentially through existing PPI networks of the taskforce members, and the European Working Group for People with Dementia (EWGPWD) and European Dementia Carers Working Group (EDCWG) (2025-2027).
Deliverables: 2024/2025
- Care Pathways SIG:
- Content analysis on (online) resources for young carers (in progress).
- Delphi study to establish recommendations for age-appropriate care pathways in YOD (in progress).
- Online support SIG:
- Opinion article on online support in YOD (submitted for publication).
Frequency of planned meetings
Online meetings two times per year. Additionally, in-person meetings will be organized at least one time a year, around major conferences (e.g. IPA and AE). We will also accommodate more frequent separate meetings for the SIG’s within the taskforce, after these have been established.
References
- Hendriks, S., et al., Global Prevalence of Young-Onset Dementia: A Systematic Review and Meta-analysis. JAMA Neurol, 2021.
- Bakker, C., M. Verboom, and R. Koopmans, Reimagining Postdiagnostic Care and Support in Young-Onset Dementia. J Am Med Dir Assoc, 2021.
- O’Malley, M., et al., Receiving a diagnosis of young onset dementia: a scoping review of lived experiences. Aging Ment Health, 2021. 25(1): p. 1-12.
- Millenaar, J.K., et al., The Impact of Young Onset Dementia on Informal Caregivers Compared with Late Onset Dementia: Results from the NeedYD Study. Am J Geriatr Psychiatry, 2016. 24(6): p. 467-74.
- Chirico, I., et al., Family experience of young-onset dementia: the perspectives of spouses and children. Aging Ment Health, 2022. 26(11): p. 2243-2251.
- Bruinsma, J., et al., The quality of the relationship perceived by spouses of people with young-onset dementia. Int Psychogeriatr, 2020: p. 1-10.
- Bodde, H.E., J.M. Papma, and J.M. Poos, Disentangling factors that influence the spousal relationship of people with young-onset dementia: starting points for person-centered care and support? Int Psychogeriatr, 2024: p. 1-8.
- Loi, S.M., M. Cations, and D. Velakoulis, Young-onset dementia diagnosis, management and care. Med J Aust, 2023. 219(2): p. 90.
- Bielderman, A., et al., Evaluation of the SPAN intervention for people living with young-onset dementia in the community and their family caregivers: a randomized controlled trial. Aging Ment Health, 2024. 28(2): p. 275-284.
- Gerritzen, E.V., McDermott, O., & Orrell, M. (2023). Online peer support: views and experiences of people with Young Onset Dementia (YOD). Aging Ment Health, 2023. 27(12). p 2386-2394
- Bruinsma, J., et al., Tailoring and evaluating the web-based ‘Partner in Balance’ intervention for family caregivers of persons with young-onset dementia. Internet Interv, 2021. 25: p. 100390.
Members
- Britt Appelhof – Radboud University Medical Center, Nijmegen, The Netherlands
- Christian Bakker – Radboud University Medical Center, Nijmegen, The Netherlands (lead)
- Sara Bartels – Maastricht University, The Netherlands
- Hanna Bodde – Erasmus Medical Center, Rotterdam, The Netherlands
- Janet Carter – University College London, United Kingdom
- Rabih Chatat – University of Bologna, Italy
- Laura Cole – University of West London, United Kingdom
- Charles David – Maastricht University, The Netherlands
- Esther Gerritzen – University of Nottingham, United Kingdom (co-lead)
- Vaitsa Giannouli – Helenic Open University , Greece
- Kate Gridly – University of York, United Kingdom
- Stevie Hendriks – Maastricht University, The Netherlands
- Rianne de Heus – Radboud University Medical Center, Nijmegen, The Netherlands
- Caitlin Hibbs – Maastricht University, The Netherlands
- Aysegul Kafadar – University of Nottingham, The Netherlands
- Tibor Kovacs – Semmelweis University, Hungary
- Raymond Koopmans – Radboud University Medical Center, Nijmegen, The Netherlands
- Hanne Kristensen – UCL University College, Denmark
- Laura Lebec – University of West Scotland, United Kingdom
- Samantha Loi – University of Melbourne, Australia
- Patricia Masterson Algar – Bangor University, United Kingdom
- Orii McDermott – University of Nottingham, United Kingdom
- Jan Oyebode – University of Bradford, United Kingdom
- Janne Papma – Erasmus Medical Center, Rotterdam, The Netherlands
- Jackie Parkes – University of Northampton, United Kingdom
- Hanne Peoples – University College London, United Kindom
- Jackie Poos – Erasmus Medical Center, Rotterdam, The Netherlands
- Louise Ritchie – University of West Scotland, United Kingdom
- Maud Ritzen – Maastricht University, The Netherlands
- Bo Smeets – Maastricht University, The Netherlands
- Catherine Talbot – Bournemouth University, United Kingdom
- Marianna Tsatali – Greek Alzheimer Association, Greece
- Rose Vincent – University of Edinburgh, United Kingdom
- Marjolein de Vugt – Maastricht University, The Netherlands
- Rachel Watson – Dementia UK, United Kingdom
- Emma Wolverson – University of Hull & Dementia UK, United Kingdom
- Natascha Woodstoke – University of West of England, United Kingdom
Palliative and End of Life Care in Dementia
Leads: Emma Wolverson, University of West London emma.wolverson@uwl.ac.uk; Karen Harrison Dening, Dementia UK Karen.Harrison-Dening@dementiauk.org; Jenny van der Steen, Leiden University Medical Center, Leiden, The Netherlands; Radboud university medical center/Radboudumc Alzheimer Center, Nijmegen, The Netherlands j.t.van_der_steen@lumc.nl
Contact person emma.wolverson@uwl.ac.uk
Date TF began: May 2023
Updated: July 2025
Background:
The number of people with dementia who have palliative care needs continues to increase and globally dementia is now the seventh leading cause of death among all diseases 1. The leading cause of death varies across countries, for example, in the UK dementia is the leading cause of death2. There is growing interest in palliative and end of life care in dementia including how to empower better care across settings 3 and in developing recommendations to support optimal care4. This taskforce will look to provide direction by identifying research priorities that have the potential for developing evidence-based support.
Aims (updated July 2025):
- To systematically review the evidence base related to psychosocial and spiritual support for those with advanced dementia and consider whether ‘Domain 8. Psychosocial and spiritual support’ of the EAPC White Paper 4 defining optimal Palliative care in older people with dementia requires updating.
- To map palliative care provision for people with dementia across different European countries within our taskforce.
- To map assisted dying legislation across European countries as it relates to people with dementia, and examine the training and support provided to healthcare professionals in responding to questions from individuals with dementia and their families.
Activities:
| Activity planned | Proposed Timeline | Update March 2024 |
| To publish the taskforces systematic review on spiritual care interventions | December 2025 | |
| To publish an opinion piece or editorial considering whether ‘Domain 8. Psychosocial and spiritual support’ of the EAPC White Paper defining optimal Palliative care in older people with dementia requires updating | October 2025- Jan 2026 | Achieved invites sent to all INTERDEM members listing interests in palliative and end of life care. Invite also circulated via INTERDEM newsletter. We remain open to new members joining. |
| To publish a paper with the findings from our mapping of palliative care provision. To develop infographics to support the dissemination of the findings.
|
October 2025- Jan 2026 | Ongoing. All members have completed a brief online survey of interests and expertise – the aim to create a narrative CV for the taskforce. |
Planned meetings:
The Taskforce has agreed to meet every two months.
References:
-
- World Health Organisation (2022). Dementia: Key Facts. Retrieved on 22/11/22 from Dementia (who.int)
- Office of National Statistics (2021). Leading causes of death, UK: 2021-2018, retrieved from Leading causes of death, UK – Office for National Statistics (ons.gov.uk)(Accessed 27 April 2023).
- Sampson, E.L., Anderson, J.E., Candy, B., Davies, N., Ellis‐Smith, C., Gola, A., Harding, R., Kenten, C., Kupeli, N., Mead, S. and Moore, K.J., 2020. Empowering better End‐of‐Life dementia care (EMBED‐Care): a mixed methods protocol to achieve integrated person‐centred care across settings. International Journal of Geriatric Psychiatry, 35(8), pp.820-832.
- Van der Steen, J.T., Radbruch, L., Hertogh, C.M., de Boer, M.E., Hughes, J.C., Larkin, P., Francke, A.L., Jünger, S., Gove, D., Firth, P. and Koopmans, R.T., 2014. White paper defining optimal palliative care in older people with dementia: a Delphi study and recommendations from the European Association for Palliative Care. Palliative medicine, 28(3), pp.197-209.
Taskforce members
- Zeena Aldridge (NHS Norfolk and Waveney ICB, UK),
- Arleen Astell (Northumbria University, UK),
- Tofunmi Aworinde (King’s College London, UK),
- Tamara Backhouse (University of East Anglia, UK) ,
- Ana Barbosa (Bradford University, UK),
- Lieve Van den Block (Vrije Universiteit Brussel, Belgium),
- Alice Burnand (University of West London, UK),
- Rozemarijn Van Bruchem-Visser (Erasmus MC, Netherlands),
- Rachel Daly (Dementia UK, UK),
- Nathan Davies (UCL, UK),
- Natashe Lemos Dekker (Leiden University, Netherlands),
- Charless Dupont (Vrije Universiteit Brussel, Belgium),
- Marlise van Eersel (Universitair Medisch Centrum Groningen, Netherlands),
- Siren Eriksen (Norwegian Advisory unit on Ageing and Health / VID Spesialized University, Norway),
- Alys Griffiths (Sheffield University, UK),
- Aysegul Kafadar (Nottingham University, UK),
- Racheal Kelley (Leeds Beckett University, UK),
- Raymond Koopmans (Radboud University Medical Cente, Netherlands),
- Jean-Bernard Mabire (Fondation Mederic Alzheimer, France),
- Rhoda Macrae (University of the West of Scotland, UK),
- Andreia Fonseca De Paiva (Bradford University, UK)
- Louise Robinson (Newcastle University, UK),
- Anne Marie Mork Rokstad (Norwegian National Advisory Unit on Ageing and Health and Molde University, Norway),
- Serena Sabatini (University of Surrey, UK),
- Hanneke Samlling (Leiden University, Netherlands),
- Edison Vidal (Sao Paulo State University, Brazil),
- Bryony Waters-Harvey (Sheffield University, UK),
- Su de Wolf-Linder (Zürcher Hochschule für Angewandte Wissenschaften, Switzerland)
Artificial Intelligence and Assistive Technology
Leads: David Neal, Amsterdam University Medical Centre, Amsterdam, the Netherlands d.n.neal@amsterdamumc.nl; Chris Fox, University of Exeter, UK
Contact: David Neal d.n.neal@amsterdamumc.nl
Established: 2012; Annual INTERDEM meeting, London UK
Updated: August 2025
Background:
Work towards understanding the contribution of digital Assistive Technologies (AT) in dementia care has gathered momentum in recent years. In 2025 this taskforce updated its seminal review on the state of the art of AT, focussing on: managing everyday life; participating in pleasurable meaningful activities; dementia care provision; and identifying gaps in the evidence and challenges for future research.1,2
Various challenges have been acknowledged.1 These include:
- Insufficient development of applications that can be easily personalised, and a lack of evidence on applications of AI in the context of dementia;
- Limited use of standardized methods to provide evidence of usability;
- Insufficient evidence of effectiveness or cost-effectiveness from randomized controlled trials (RCTs) or alternative forms of robust evaluation;
- Implementation barriers in relation to overly high expectations for AT;
- From an ethical perspective, a need for more equitable access to technologies.
These challenges around AT can be categorized as implementation gaps, evidence gaps and needs gaps.
INTERDEM has supported collaborative pan-European research into AT, including two programmes funded by EU HORIZON 2020 Marie Sklodowska-Curie Actions, INDUCT and DISTINCT. These networks aimed to develop a multi-disciplinary, inter-sectorial educational research framework for Europe to improve technology and care for people with dementia and to provide the evidence to show how technology can improve the lives of people with dementia. 3 A number of publications in peer review journals have arisen from INDUCT and DISTINCT, providing new insights towards bridging implementation, evidence and needs gaps, and some of its work was published in a recent book.4,5 DISTINCT covered three themes, namely: technology to fulfil potential and obligations at a societal level; technology to manage one’s own life; and technology enabling participation in social activities.6 Despite these successes, INDUCT and DISTINCT have only scratched the surface: major implementation, evidence and needs gaps remain.
Therefore between 2023 and 2025 we refreshed our aims and completed the following work programmes.
- Laid the groundwork for an update to the taskforce’s 2017 position paper based on a new review of systematic reviews and Delphi study.1,2,7
- Supported the JPND INTEREST network resulting in two peer-revied publications. One publication reviewed the current state of dementia care guidelines in Europe, specifically with respect to equity and the place of technology.8 The second paper reviewed the availability of personalised, effective and usable AT, based on existing literature.9
- Published article: determinants and strategies of (un)successful implementation of assistive technologies in dementia care: an explorative survey in Europe.10
- Completion of research project and publication of article: evidence synthesis relating to the uptake of AT among people with dementia during the COVID-19 pandemic. Finalizing consensus recommendations from this project.11
Current work (2025/2026):
In 2025, artificial intelligence (AI) has been added as a focus of the taskforce. There is great hype surrounding AI, both in general in society and in health and care, and applications of generative or predictive AI could theoretically be integrated with a very wide range of existing digital AT. However, our review of systematic reviews identified no research addressing the use of AI in dementia care.1 Given the potential challenges surrounding integration of AI into AT for people living with dementia, especially in relation to development, implementation and ethics, it is therefore important that this taskforce facilitates high quality research in this emerging field.
Workplans 2025/2026:
- The systematic review of systematic reviews has now been published 1 ( see https://journals.sagepub.com/doi/10.1177/20552076251362353 ). Once the Delphi study is published, we aim to write a white paper (or position paper) based on the findings from both studies.
- A working group of the taskforce will be formed to support dissemination of the position paper and underlying research to professionals, policymakers, and other stakeholders.
- Further work plans, and the establishment of working groups to execute these plans and gaps surrounding AI, will be discussed at the INTERDEM annual meeting in Bologna, in October 2025.
Frequency of Planned Meetings:
This Taskforce generally meets four times per year online.
Additionally, we organise in-person taskforce meetings around major conferences. For example, in 2023, in-person meetings took place at the International Psychogeriatric Association congress in Lisbon, in June, and at the Alzheimer Europe conference in Helsinki, in October and we met in person at the Alzheimer Europe conference in Geneva in 2024. In 2025 we will meet in person at the Alzheimer Europe conference in October.
References:
- Neal, D., Craven, M., Cross, J., Evans, S., Fox, C., Oksnebjerg, L., Alexandre, I., Aryankhesal, A., Astell, A., Ahmet B., Ditton, A., Engelsma, T. , Gregersen, R., Heins, P., Hogervorst, E., Kafadar, E.H., Poos, J., Robinson, L., Sezgin. D., Smaling, H., Szczesniak, D., Tan, J.R., de Vugt, M. and Meiland, F.M. (2025). Digital assistive technologies for community-dwelling people with dementia: A systematic review of systematic reviews by the INTERDEM AI & assistive technology taskforce. Digital health https://journals.sagepub.com/doi/10.1177/20552076251362353
- Meiland F, Innes A, Mountain G, Robinson L, van der Roest H, García-Casal JA, Gove D, Thyrian JR, Evans S, Dröes R, Kelly F, Kurz A, Casey D, Szcześniak D, Dening T, Craven MP, Span M, Felzmann H, Tsolaki M, Franco-Martin M. Technologies to Support Community-Dwelling Persons With Dementia: A Position Paper on Issues Regarding Development, Usability, Effectiveness and Cost-Effectiveness, Deployment, and Ethics JMIR Rehabil Assist Technol 2017;4(1): e1 doi: 2196/rehab.6376
- INDUCT: https://www.dementiainduct.eu/
- Orrell M, Oliveira D, McDermott O, Verhey FR, Dassen FC, Dröes RM, editors. Improving the lives of people with dementia through technology: interdisciplinary network for dementia utilising current technology. Taylor & Francis; 2022 Nov 30.
- DISTINCT: https://www.dementiadistinct.com/;
- DISTINCT projects: ESRs Projects – DISTINCT (dementiadistinct.com)
- Sezgin, D., Hegerath, F-M., Christie, H., Poos, J., Cullen, K., Meagher, E., Goncalves Pereira, M., Vollmar, H.C., O’Reilly, C., Mitchell, A., Alabdulkder, S., Neal, D.P., Janus, S. Recommendations for the development and use of technology to support people living with dementia and their caregivers: A Delphi study. In review.
- Neal, D., Bartels, S.L., Berdai Chaouni, S., Caprioli, T., Comas-Herrera, A., Chattat, R., Diaz, A., Dröes, R.M., Faulkner, T., Felding, S.A. and Franco-Martin, M., 2025. Effective for Whom? A Review of Psychological and Social Intervention Recommendations in European Dementia Care Guidelines Through the Lenses of Social Health and Intersectionality. Behavioral Sciences, 15(4), p.457. https://doi.org/10.3390/bs15040457
- Szczesniak, D., Molinari-Ulate, M., Neal, D.P., Bartels, S.L., Diaz Baquero, A.A., Thyrian, J.R., Franco Martin, M., Wolf-Ostermann, K., Rymaszewska, J., Dröes, R-M. Toward Better Technology for Social Health in Dementia: A Review of Implementation Recommendations and Research Priorities to Equitably meet the Demand. In preparation.
- Roest H.G., Christie H.L., Franco-Martín M.A., Dröes R-M., de Vugt M.E., Meiland F.J.M.(2024) Determinants of (un)successful implementation of research-based assistive technologies in dementia care: an explorative survey. JMIR Aging 2024;7: e53640 doi: 10.2196/53640
- Barbosa A., Ferreira A.R., Smits C., Hegerath F.M., Vollmar H.C., Fernandes L., Craven M.P., Innes A., Casey D., Sezgin D., Hopper L., Øksnebjerg L. Use and uptake of technology by people with dementia and their supporters during the COVID-19 pandemic. Aging Ment Health. 2024 Jan-Feb;28(1):83-94. doi: 10.1080/13607863.2022.2163375
Members (currently 67 members)
Isabel Alexandre, University Institute of Lisbon, Portugal; Golnaz Atefi, Radboud University Medical century, The Netherlands; Arlene Astell, University of Reading, UK; Ana Barbosa, University of Bradford, UK; Ellis Bartholomeous, Eindhoven Technical University, The Netherlands; Ahmet Begde, Loughborough University, UK; Jeroen Bruinsma, Maastricht University, The Netherlands; Julieta Camino, University of East Anglia, UK; Rabih Chattat, University of Bologna, Italy; Ilaria Chirico, University of Bologna, Italy; Hannah Christie, Maastricht University, The Netherlands; Michael Craven, University of Nottingham, UK; Jane Cross, University of East Anglia, UK; Tom Dening, University of Nottingham, UK; Priscilla Doyle, University of Galway, Ireland; Rose-Marie Dröes, Amsterdam UMC (VUmc), The Netherlands; Thomas Engelsma, Amsterdam UMC, The Netherlands; Shirley Evans, University of Worcester, UK; Katie Featherstone, University of West London, UK; Simone Felding, DZNE, Germany; Lia Fernandes, University of Porto, Portugal; Ajda Flisar, KU Leuven, Belgium; Chris Fox, University of Exeter, UK (co-lead); Manuel Franco, Intras, Spain; Lesley Garcia, University of Nottingham, UK; Vaitsa Giannouli, Hellenic Open University, Greece; Manuel Gonçalves-Pereira, Nova Medical School, Portugal; Dianne Gove, Alzheimer Europe, Luxembourg; Rikke Gregersen, VIA University College, Denmark; Kyle Harrington, the University of Nottingham, UK; Pascale Heins, Maastricht University, The Netherlands; Eef Hogervorst, Loughborough University, UK; Louise Hopper, Dublin City University, Ireland; Maarten Houben, TU Eindhoven, the Netherlands; Anthea Innes, McMaster University, Canada; Sarah Janus, UMCG Groningen, The Netherlands; Aysegul Kafada, University of Nottingham, UK; Raymond Koopmans, Radboud UMC, Nijmegen, The Netherlands; Jenni Lynch, University of Hertfordshire, UK; Esther Loseto-Gerritzen, University of Nottingham, UK; Franka Meiland, Amsterdam UMC (VUmc), The Netherlands; Mauricio Molinari, University of Salamanca, Spain; Esme Moniz-Cook, University of Hull, UK; David Neal , UMC Amsterdam, The Netherlands (co-lead); Laila Øksnebjerg, University of Copenhagen, Denmark; Giovanni Ottoboni, University of Bologna, Italy; Channah Osinga, Amsterdam UMC, The Netherlands; Annette Plantinga, NHL Stenden University and the Alzheimer Center of Groningen, The Netherlands; Jackie Poos, Erasmus UMC, The Netherlands; Anne Margriet Pot, WHO, Switzerland / Erasmus UMC, The Netherlands; Louise Robinson, Newcastle University, UK; Anthony Scerri, University of Malta, Malta; Duygu Sezgin, University of Galway, Ireland; Sietske Sikkes, Amsterdam UMC, The Netherlands; Hanneke Smaling, Leiden University MC, The Netherlands; Sarah Smith, Leeds Beckett University, UK; Dirk Steijger, Maastricht University, The Netherlands; Dorota Szcześniak, Wroclaw Medical University, Poland; Josephine Tan, Amsterdam UMC, The Netherlands; Daksha Trivedi, University of Hertfordshire, UK; Henriette van der Roest, Amsterdam UMC (VUmc), The Netherlands; Horst Vollmar, Ruhr-University Bochum, Germany; Kay de Vries, The Gateway, De Montfort University, UK; Marjolein de Vugt, Maastricht University, The Netherlands; Tracy Williamson, University of Worcester, UK; Emma Wolverson, University of West London, UK; Nahid Zokaei, Brain Plus, Denmark.
Social Health
Leads: Prof. Dr. Myrra Vernooij-Dassen, the Netherlands; Prof. Dr. Marjolein de Vugt, the Netherlands; Prof. Dr. Dorota Szczesniak, Poland
Contact: Myrra Vernooij-Dassen@radboudumc.nl
Updated: May 2025
Background
Dementia research has traditionally focused on biological and psychological factors, with social aspects often overlooked. However, the Social Health Taskforce recognizes dementia as a multifactorial syndrome and is committed to exploring the role of social health in both preventing cognitive decline and dementia and improving quality of life for those living with dementia and their carers. Over the past decade, INTERDEM has played a pivotal role in conceptualizing and operationalizing social health. and its definitional and measurement work. Social health refers to the relational aspect of health, reflecting the influence on each other of the individual and the social environment.
Our research has successfully bridged the gap between biological, psychological, and social research, paving the way for a more comprehensive biopsychosocial health model— and has added an eco-element to describe external influences. We’ve identified modifiable social health markers, opening up new possibilities for interventions. The taskforce also maintains strong connections with the prevention, methodology, and technology taskforces.
Key aims of the Taskforce
Conceptualizing Social Health in dementia
- Further refine the conceptual framework for social health, ensuring it captures the diverse needs and experiences of people living with dementia.
- Advance our understanding of how social health influences dementia progression and overall well-being.
Understanding mechanisms
- Investigate the mechanistic pathways linking social health with brain health, recognizing that the relationship is likely bi-directional (i.e., social engagement affects cognitive function, but cognitive decline also influences social interactions).
- Explore biological, psychological, and environmental factors that shape the impact of social health over time.
Developing and evaluating Social Health measures
- Identify and validate social health markers that can be used to track changes and evaluate interventions in dementia research.
- Ensure that measures are inclusive and adaptable to different cultural and social contexts.
Interventions: What works for Whom?
- Move beyond a ‘one-size-fits-all’ approach to tailor interventions to the diversity of dementia (e.g., different subtypes, stages, and personal needs).
- Examine the mechanisms of action behind social health interventions
- Address the specific needs of underrepresented groups by integrating insights from equity and intersectionality research
Strengthen international collaboration
- Strengthen the international research network within INTERDEM to drive forward innovations in social health.
- Share best practices, research findings, and interventions across countries to optimize the global impact of social health research in dementia.
Future Directions
Prevention
- More evidence needed on relationship social and brain health
- Inclusion of social health in preventive interventions
- Implementation knowledge on impact
Treatment and care
- Examine how social health changes across the dementia trajectory and its impact on brain health, care needs, quality of life, and disease progression.
- Identify and validate social health markers that can be used to personalize interventions and improve person-centered care.
- Explore Digital and AI-based technologies as tools to enhance social health, particularly for individuals with limited access to in-person interactions.
- Ensure that policies, interventions, and technologies are aligned with promoting social health in dementia.
Activities for 2025:
- Organisation of symposia (Alzheimer Europe, IPA)
- Publications relating to social health and dementia
- Collaborate in research proposals and projects, also involving early career researcher from the INTERDEM Academy
Project group: Measurement social health
Myrra Vernooij-Dassen
Karin Wolf-Ostermann
Debby Gerritsen Noortje Kloos: noortje.kloos@radboudumc.nl
Sara Bartels
Charlotte Stoner
Emma Wolverson Pascale Heins: p.heins@maastrichtuniversity.nl
Esme Moniz-Cook
Marjolein de Vugt
Sietske Sikkes
Dorota Szeszcniak Juliety Camino: j.camino@uea.ac.uk
Frequency of Meetings: Three times per year
References:
- Huber M, Knottnerus JA, Green L, van der Horst H. et al. (2011) How should we define health? Jul26;343:d4163. doi: 10.1136/bmj.d4163. PMID: 21791490.
- Vernooij-Dassen M, Jeon Y-H. ( 2016) Social health and dementia: the power of human capabilities. International psychogeriatrics. 2016;28(5):701-3.
- Dröes R, Chattat R, Diaz A, Gove D, Graff M, Murphy K, et al. ( 2017) Social health and dementia: a European consensus on the operationalization of the concept and directions for research and practice. Aging & mental health. 2017;21(1):4-17.
- de Vugt M, Dröes RM. ( 2017) Social health in dementia. Towards a positive dementia discourse. Aging Ment Health. (1): 1-3. doi: 10.1080/13607863.2016.1262822. PMID: 28098498.
- Vernooij-Dassen M, Moniz-Cook E, Jeon YH. ( 2018) Social health in dementia care: harnessing an applied research agenda. International Psychogeriatrics.30(6):775-8.
- Stiekema, A. P. M., van Heugten, C. M., & de Vugt, M. E. ( 2019) Joining forces to improve psychosocial care for people with cognitive deficits across diagnoses: social helath as a common framwork Aging Ment Health. 10, 1275-1281. doi: 10.1080/13607863.2018.1498446.
- Vernooij-Dassen M, Verhey F, Lapid M. (2020) The risk of social distancing for older adults: a call to balance. Int Psychogeriatr 10):1235-1237. doi: 10.1017/S1041610220001350. Epub 2020 Jun 24.PMID: 32576306
- Heins, P., Boots, L. M., Koh, W. Q., Neven, A., Verhey, F. R., & de Vugt, M. E. (2021). The effects of technological interventions on social participation of community-dwelling older adults with and without dementia: A systematic review. Journal of clinical medicine, 10(11), 2308.
- Vernooij-Dassen M, Moniz-Cook E, Verhey F, Chattat R, Woods B, Meiland F, et al. (2021) Bridging the divide between biomedical and psychosocial approaches in dementia research: the 2019 INTERDEM manifesto. Aging Ment Health. 2021;25(2):206-12.
- Duffner, L. A., Deckers, K., Cadar, D., Steptoe, A., De Vugt, M., & Köhler, S. (2022). The role of cognitive and social leisure activities in dementia risk: assessing longitudinal associations of modifiable and non-modifiable risk factors. Epidemiology and Psychiatric Sciences, 31, e5. Epidemiol Psychiatr Sci. 31:e5. doi: 10.1017/S204579602100069X
- Vernooij-Dassen M, Verspoor E, Samtani S, Sachdev PS, Ikram MA, Vernooij MW, et al. (2022) Recognition of social health: A conceptual framework in the context of dementia research. Front Psychiatry. 13:1052009.
- Samtani S, Mahalingam G, Lam BCP, Lipnicki DM, Lima-Costa MF, Blay SL, et al. Associations between social connections and cognition: a global collaborative individual participant data meta-analysis. Lancet Healthy Longev. 2022;3(11):e740-e53.
- van der Velpen IF, Melis RJF, Perry M, Vernooij-Dassen MJF, Ikram MA, Vernooij MW (2022). Social Health Is Associated With Structural Brain Changes in Older Adults: The Rotterdam Study. Biol Psychiatry Cogn Neurosci Neuroimaging. 7(7):659-68.
- Freak-Poli R, Wagemaker N, Wang R, Lysen TS, Ikram MA, Vernooij MW, Dintica CS, Vernooij-Dassen M, Melis RJF, Laukka EJ, Fratiglioni L, Xu W, Tiemeier H.J (2022). Loneliness, not social support, is associated with cognitive decline and dementia across two longitudinal population-based cohorts. Alzheimers Dis. 85(1):295-308. doi: 10.3233/JAD-210330.PMID: 34842183
- Duffner, L. A., DeJong, N., Jansen, J. F., Backes, W., de Vugt, M., Deckers, K., & Köhler, S. (2023). Associations between social health factors, cognitive activity and neurostructural markers for brain health –a systematic literature review and meta-analysis. Ageing Res Rev. Jun 24;89:101986. doi: 10.1016/j.arr.2023.101986. Epub ahead of print. PMID: 37356551.
- Duffner, L., Deckers, K., Cadar, D., Steptoe, A., de Vugt, M., & Köhler, S. (2022). Social health factors and dementia risk–assessing potential pathways in a population‐based cohort study. Alzheimer’s & Dementia, 18, e065441. https://doi.org/10.1002/alz.065441
- Lenart-Bugla M, Łuc M, Pawłowski M, Szcześniak D, Seifert I, Wiegelmann H, Gerhardus A, Wolf-Ostermann K, Rouwette EAJA, Ikram MA, Brodaty H, Jeon YH, Maddock J, Marseglia A, Melis RJF, Samtani S, Wang HX, Welmer AK, Vernooij-Dassen M, Rymaszewska J (2022).What do we know about social and non-social factors influencing the pathways from cognitive health to dementia? A systematic review of reviews. Brain Sci. 12(9):1214. doi: 10.3390/brainsci12091214.PMID:
- Costanzo A, van der Velpen IF, Ikram MA, Vernooij-Dassen MJF, Niessen WJ, Vernooij MW, Kas MJ (2022). Social health is associated with tract-specific brain white matter microstructure in community-dwelling older adults. Biol Psychiatry Glob Open Sci;3(4):1003-1011. doi: 10.1016/j.bpsgos.2022.08.009. eCollection 2023 Oct.PMID: 37881589
- Seifert I, Wiegelmann H, Lenart-Bugla M, Łuc M, Pawłowski M, Rouwette E, Rymaszewska J, Szcześniak D, Vernooij-Dassen M, Perry M, Melis R, Wolf-Ostermann K, Gerhardus A; SHARED consortium (2022). Mapping the complexity of dementia: factors influencing cognitive function at the onset of dementia.BMC Geriatr. (1):507. doi: 10.1186/s12877-022-02955-2.PMID: 35725402
- Maddock J, Gallo F, Wolters FJ, Stafford J, Marseglia A, Dekhtyar S, Lenart-Bugla M, Verspoor E, Perry M, Samtani S, Vernooij-Dassen M, Wolf-Ostermann K, Melis R, Brodaty H, Ikram MA, Welmer AK, Davis D, Ploubidis GB, Richards M, Patalay P; SHARED Consortium (2023). Social health and change in cognitive capability among older adults: findings from four European longitudinal studies. 69(11):1330-1346. doi: 10.1159/000531969. Epub 2023 Jul 27.PMID: 37497894
- Mahalingam G, Samtani S, Lam BCP, Lipnicki DM, Lima-Costa MF, Blay SL, Castro-Costa E, Shifu X, Guerchet M, Preux PM, Gbessemehlan A, Skoog I, Najar J, Sterner TR, Scarmeas N, Yannakoulia M, Dardiotis T, Kim KW, Riedel-Heller S, Röhr S, Pabst A, Shahar S, Numbers K, Ganguli M, Hughes TF, Chang CH, Crowe M, Ng TP, Gwee X, Chua DQL, Rymaszewska J, Wolf-Ostermann K, Welmer AK, Stafford J, Mélis R, Vernooij-Dassen M, Jeon YH, Sachdev PS, Brodaty H; SHARED consortium for the Cohort Studies of Memory in an International Consortium (COSMIC) (2023). Social connections and risk of incident mild cognitive impairment, dementia, and mortality in 13 longitudinal cohort studies of ageing). Alzheimers Dement. 19(11):5114-5128. doi: 10.1002/alz.13072. Epub 2023 Apr 27.PMID: 37102417
- Altona J, Wiegelmann H, Lenart-Bulga M, Vernooij-Dassen M, Verspoor E, Seifert I, Misonow J, Szcześniak D, Rymaszewska J, Chattat R, Jeon YH, Moniz-Cook E, Roes M, Perry M, Wolf-Ostermann K (2024). Instruments for assessing social health in the context of cognitive decline and dementia: systematic review.Front Psychiatry. 2024 Nov 13;15:1387192. doi: 10.3389/fpsyt.2024.1387192. eCollection 2024.PMID: 39605998
- Kristanti MS, Vernooij-Dassen M, Jeon YH, Verspoor E, Samtani S, Ottoboni G, Chattat R, Brodaty H, Lenart-Bugla M, Kowalski K, Rymaszewska J, Szczesniak DM, Gerhardus A, Seifert I, A’la MZ, Effendy C, Perry M (2024). Social health markers in the context of cognitive decline and dementia: an international qualitative study. Front Psychiatry. 15:1384636. doi: 10.3389/fpsyt.2024.1384636. eCollection 2024.PMID: 39364383
- van der Velpen IF, Yaqub A, Vernooij MW, Perry M, Vernooij-Dassen MJF, Ghanbari M, Ikram MA, Melis RJF (2024). Sex-differences in the association of social health and marital status with blood -based immune and neurodegeneration markers in a cohort of community-dwelling older adults. Brain Behav Immun. 81. doi: 10.1016/j.bbi.2024.05.031. Epub 2024 May 21.PMID: 38782212 van der Velpen IF, Melis RJF, Hussainali RF, Perry M, Vernooij-Dassen MJF, Ikram MA, Luik AI, Vernooij MW (2024). Determinants of social health trajectories during the COVID-19 pandemic in older adults: the Rotterdam study. Int Psychogeriatr(8):628-642. doi: 10.1017/S1041610221002891. Epub 2022 Jan 28.PMID: 35086605
- Casey, D., Comas-Herrera, A., Diaz, A., Hopper, L., Karl, J. A., Koh, W., … Thyrian, J. R. (2024, April 15). Exploring unmet needs and inequity among people affected by dementia using an intersectionality lens: A scoping review of reviews. https://doi.org/10.17605/OSF.IO/4H8ME
- Vernooij-Dassen M, van der Velpen IF, Lanooij SD, van der Zee EA, Ikram MA, Drinkenburg WHIM, Costanzo A, Vernooij MW, Eisel ULM, Melis R, Kas MJH, Perry M. Social health and prevention of dementia: integration of human and mice studies. (2025 ).Int Psychogeriatr. 100054. doi: 10.1016/j.inpsyc.2025.100054. Online ahead of print.PMID: 40050164
Task Force Members:
Astell, Arlene; Bakker, Christian; Bartels, Sara; Casey, Dympna; Chattat Rabih; Craven, Michael; Davies-Abbott, Ian; Deckers, Kay; Diaz, Ana; Dröes, Rose-Marie; Dunn, Rosie; Evans, Shirley; Fernandes, Lia; Fonseca de Paiva, Andreia; Forstmeier, Simon; Garcia, Lesley; Gerritsen, Debby; Gerritzen, Esther; Giebel, Clarissa; Gonçalves-Pereira, Manuel; Gove, Dianne; Graff Maud; Harrington, Kyle; Heins, Pascale; Hoe, Juanita; Hopper, Louise; Hout Hein van; Hudson, John; Irving, Kate; Johannessen, Aud; Keady John; Köhler, Sebastian; Kristensen, Fritze; Laporte-Uribe, Franziska; Lenart, Marta; Lion, Katarzyna; Machado Alexandre, Isabel; Mc Hugh, Joanna; Moniz-Cook Esmé; Neal, David; Oeksnebjerg, Laila; Oliveira, Deborah Christina de; Perry, Marieke; Rafnsson, Snorri Bjorn; Reilly, Siobhan; Roes, Martina; Stoner, Charlotte; Surr, Claire; Szczesniak, Dorota; Tan, R.J. Tatzer, Verena; Välimäki, Tarja; Verbeek, Hilde; Vernooij-Dassen, Myrra; Vugt de, Marjolein; Waters, Bryony; Wardt, Veronika van der; Wilcock, Jane; Wolf, Karin; Wolverson, Emma.
Methodology
Novel designs and methodologies for complex intervention research in dementia care
Lead: Maud Graff, Radboud University Medical Center, Nijmegen, The Netherlands
Co-Leads: Sara Laureen Bartels, Alzheimer Centrum Limburg, Maastricht University, Maastricht, The Netherlands; Federica D’Andrea, The Geller Institute of Ageing and Memory (GIAM), University of West London, UK
Contact: Maud Graff Maud.graff@radboudumc.nl
Established: 2007 Estoril, Portugal: renewed 2018 at Alzheimer Europe INTERDEM meeting Barcelona, Spain and INTERDEM meeting (Bucharest) 2022. New Leadership (2023).
Updated: July 2025
Introduction
This taskforce was initiated early in the life of INTERDEM with funded projects and publications on outcome measurements (2003-4; JPND Charting new territory 2014 -2015), quality indicators (EUROCODE (2006 -2008), methodological issues and on involving people with dementia in research. For information on this work and a full background rationale for the present Taskforce see Appendix: Background and References 1 – 25.
New leadership in 2023 facilitated discussions about knowledge on how to design inclusive psychosocial intervention research which we agreed was a next step for advancing methodology in dementia care research. This also has scope to raise awareness of alternative research methods for use by funders, reviewers and journal editors.
Overall Task Force Aims
- To provide an overview of relevant new designs, methodologies and approaches to address differing research questions, in the development evaluation and implementation of psychosocial interventions in dementia research – that fits to routine practice, political and societal expectations, and cover MRC core elements;
- To map and gain consensus on designs, research methodologies and approaches to address differing research aims in psychosocial intervention dementia research, and that cover the MRC core elements; and place them in, between or alongside the different phases of the current UK MRC framework (Skivington et al. 2021).
- To disseminate knowledge on current research practices and future directions for methodology in psychosocial intervention dementia research work – as relevant to researchers, policy makers, people with dementia, carers, professionals and other stakeholders.
Frequency of meetings: Monthly (second Thursday of each month)
Activities and Deliverables 2023 – 2026
- organise discussion with INTERDEM researchers and beyond on placing relevant (new) methodologies and approaches in, between or alongside phases of the current MRC framework (Skivington et al. This began in 2023 and remains ongoing work (i) during meetings with TF members presenting on different topics; see Deliverables (i) 1-10 below – TF presentations and our published opinion paper (see References Bartels et al. 2024).
- disseminate and provide opportunities for new learnings with audiences internationally and with early career researchers – now incorporated in our 2-year funded taskforce research study METHODEM: Methodological consensus for complex Interventions in dementia, evaluating or implementing psychosocial interventions research in dementia. This was submitted by Maud Graff and Sara Bartels and is now led by Maud Graff, Sara Bartels and Federica D’Andrea (TF lead/co- leads) with active collaboration from TF members. We use engagement workshops / discussions surrounding our research award (see Deliverables (ii) 1 -14 below – webinars and presentations and a current submission (Bartels et al. 2025). The focus is on the METHODEM research programme involving a systematic scoping review (see submitted protocol D’Andrea et al. 2025) where TF members are helping with screening 23,079 articles; and an online Delphi study to collect relevant (new) approaches to designs and methodologies. This work will continue into 2025/ 2026.
- move from our opinion paper (Bartels et al., 2024) to publish a systematic scoping review and consensus work on new methodologies and approaches related to differing research aims for psychosocial interventions in dementia research, that cover the MRC core elements – with recommendations for use within, between or alongside phases of the current MRC framework. This is as part of the METHODEM 2-year study.
References
Bartels SL, Stephens N, D’Andrea F, Handley M, Markaryan M, Nakakawa Bernal A, Van den Block L, de Bruin SR, Windle K, Roes M, Janssen N, Christie H, Garcia L, Teesing G, Moniz-Cook E and Graff M (2024) Discussing methodological gaps in psychosocial intervention research for dementia: an opinion article from the INTERDEM Methodology Taskforce guided by the MRC framework. Frontiers in dementia, 3, 1458023 Doi: 10.3389/frdem.2024.1458023
Bartels, Sara Laureen, D’Andrea, Federica, Graff, Maud – on behalf of the INTERDEM Methodology Taskforce (submitted July 2025) Advancing methodologies and methods for psychosocial intervention research in dementia: The METHODEM project. Occupational Therapist Science Column (submitted July 2025)
D’Andrea F, Bartels SL, Markaryan M., Algar PM, Nakakawa Bernal A., de Bruin SR, Chirico, Flynn A., Garcia L, Gebhard D, Handley M, Janssen N, Roes M, Stephens N, Teesing G, Van den Block L, Windle K, Moniz-Cook E. and Graff M. (submitted April 2025 ) Methodologies and methods for the development, evaluation, and implementation of psychosocial interventions for dementia: Protocol for a scoping review.
Skivington K, Matthews L, Simpson SA, Craig, P Baird B, Blazenby, JM, Boyd KA, Craig N, French DP, McIntosh E, Petticrew, M & Rycroft-Malone J. (2021) A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance, BMJ, 374:n2061 https://doi.org/10.1136/bmj.n2061
Deliverables (2023 –July 2025)
(i) Taskforce meeting presentations
- Sara Laureen Bartels: The Experience Sampling Method: Digital self-monitoring to understand and support everyday life in aging and dementia. Power Point slides bartels@maastrichtuniversity.nl
- Maud Graff: Use of different research designs for developing, evaluating & implementing person-centered psychosocial interventions in dementia – applied within the MRC framework. PowerPoint slide/related papers graff@radboudumc.nl
- Simone de Bruin: Participatory Action Research to improve dementia care and support. PowerPoint slides de.bruin@windesheim.nl
- Nathan Stephens: A pragmatic evaluation of the Worcestershire Meeting Centres Programme for people affected by dementia – Power Point slides/related papers n.j.stephens@outlook.com
- Lieve Van den Block: Theory of change to develop and evaluate complex palliative care interventions. Power Point slides van.den.block@vub.be
- Fanny Monnet: Developing a website to support advanced care planning for people with dementia and their family caregivers Power Point slides monet@vub.be
- Susan Conquer: PAR and co-production of a Library Memory Café. Power Point slides Conquer@uos.ac.uk
- Federica D’Andrea: Building a Programme Theory for olfactory stimulation interventions: a realist review. For Power Point slides/ information federica.dandrea@uwl.ac.uk
- Doris Gebhard: Insights into methods used in research on health promotion in long-term care. For Power Point slides/ information. doris.gebhard@tum.de
- Marine Markaryan, Part 1 ‘A rigorous stepwise procedure for multi-stakeholder design of implementation actions. Part 2: Maintaining Couple hood in the face of dementia’: PhD Trajectory presentation for Methodology Taskforce. For Power Point slides/ information marine.markaryan@maastrichtuniversity.nl
(ii) Webinars/ Engagement Presentations / Dissemination (March 2023 – July 2025)
- Webinar– Invitation to INTERDEMas part of a series by the LINC-AD About | LINC-AD | Alzheimer’s Association in collaboration with the GSA Maud J. L .Graff: Use of different research designs for developing, evaluating and implementing person-centered psychosocial interventions in dementia and applied within the MRC framework. GSA webinar, March 2, 2023 – Contact maud.graff@radboudumc.nl
- Webinar– Invited by the MSCA training network HOMEDEM as part of the doctoral training (November 27, 2023) Presentations by Maud J.L. Graff and Sara Laureen Bartels on “Frameworks to develop, evaluate, and implement complex interventions for people with long-term health conditions”. Contact: sara.bartels@maastrichtuniversity.nlor maud.graff@radboudumc.nl
- Webinar – INTERDEM Spotlight series, invited by INTERDEM Academy (10.30-12.30, March 8th, 2024) Presentations by Maud J.L. Graff, Lieve Van den Block, and Nathan Stephens: Innovative methodologies and designs in dementia research Contact: maud.graff@radboudumc.nl, lieve.van.den.block@vub.be, n.j.stephens@outlook.com
- Oral Presentation – 34th Alzheimer’s Europe Conference. Geneva Switzerland (October 2024) Maud, Graff, Sara Lauren Bartels and Federica D’Andrea: Designs and methodologies to develop, evaluate, and implement psychosocial interventions in dementia. Discussing gaps and advancing the field through the METHODEM project.
- Poster Presentation – 34th Alzheimer’s Europe Conference. Geneva Switzerland (October 2024) Federica D’Andrea, Sara Laureen Bartels, Nathan Stephens, members of the INTERDEM Methodology Taskforce & Maud Graff: Novel designs and methodologies for complex psychosocial interventions in dementia.
- Poster Presentation – 34th Alzheimer’s Europe Conference. Geneva Switzerland (October 2024) Sara Laureen Bartels, Federica D’Andrea, members of the INTERDEM Methodology Taskforce & Maud Graff: METHODEM: Methodological consensus for complex interventions in dementia.
- Oral Presentation – Ist Occupational Therapy Europe Congress, Kraków, Poland (October 2024) Maud Graff: Innovative methods and methodologies for developing, evaluating, and implementing occupational therapy and other psychosocial interventions in dementia. How to cover the core-elements of the MRC framework.
- In-Person workshop – SPREAD+ consortium meeting – Dutch dementia network, Amsterdam, The Netherlands (2025). Sara Laureen Bartels & members of the INTERDEM Methodology Taskforce. Measuring what matters.
- Webinar – ISTAART Non-pharmacological Intervention Professional Interest Area (PIA): Tiny Dots (May 2025) Federica D’Andrea, Maud Graff and Melanie Handley & members of the INTERDEM Methodology Taskforce: Methods and methodologies to develop, evaluate, and implement psychosocial interventions in dementia. Discussing gaps and advancing the field through the METHODEM project.
- Poster Presentation – 18Th UK Dementia Congress, Coventry, UK (November 2024). Federica D’Andrea, Sara Laureen Bartels, Nathan Stephens, members of the INTERDEM Methodology Taskforce & Maud Graff: Novel designs and methodologies for complex psychosocial interventions in dementia.
- Poster Presentation: 18Th UK Dementia Congress, Coventry, UK (November 2024). Federica D’Andrea, Sara Laureen Bartels, members of the INTERDEM Methodology Taskforce & Maud Graff: METHODEM: Methodological consensus for complex interventions in dementia
- Invited Presentation –Paris Nanterre University, Seminar (May 2025) Federica D’Andrea & , members of the INTERDEM Methodology Taskforce: Psychosocial intervention research for dementia: Discussing methodological gaps and novel approaches.
- Oral presentation – British Society of Gerontology 54th Annual Conference (June 2025). Federica D’Andrea, Sara Laureen Bartels, the INTERDEM Methodology Taskforce, & Maud Graff: Methodologies and methods for research on psychosocial interventions for dementia: Gaps and state-of-the-art evidence.
- Poster presentation – British Society of Gerontology 54th Annual Conference (June 2025): Federica D’Andrea, Sara Laureen Bartels, the INTERDEM Methodology Taskforce & Maud Graff: Consensus on core elements and methodological approaches for research on psychosocial interventions in dementia: Protocol for a Delphi study
Taskforce Members (July 2025):
Maud Graff, Radboud University Medical Center, Nijmegen, NL, graff@radboudumc.nl; (lead); Sara Laureen Bartels, Maastricht University, NL, bartels@maastrichtuniversity.nl; (co-lead); Federica D’Andrea, University of West London, UK, federica.dandrea@uwl.ac.uk (co-lead);
Members in Alphabetical order
Patricia Masterson Algar, Bangor University, Wales, UK;
Andrea Nakakawa Bernal, The Polytechnic University of Milan, Italy;
Simone de Bruin, Windesheim University of Applied Sciences, NL;
Maria Caulfield, University of Bradford, UK;
Ilaria Chirico, University of Bologna, Italy;
Emma Elliott, University of Manchester, UK;
Aisling Flynn, Bournemouth University, UK;
Lesley Garcia, University of Nottingham, UK;
Doris Gebhard, Technical University of Munich, Germany;
Melanie Handley, University of Hertfordshire, UK;
Niels Janssen, Maastricht University, NL;
NL; Marine Markaryan, Maastricht University, NL;
Esme Moniz-Cook, University of Hull, UK;
Nathan Stephens, University of Worcester, UK;
Martina Roes, DZNE/Witten, Germany;
Lieve Van den Block, Vrije Universiteit Brussel, Belgium;
Karen Windle, University of Ulster, Northern Ireland, UK.
Appendix: Background / References: 1-25 (** 11 published in 2025)
This taskforce was initiated early in the life of INTERDEM. It initially focused on outcome measurements (2003-4) and methodology1, 2. Some of its work was funded through the EUROCODE project (2006 -2008) where quality indicators were developed3 and through an INTERDEM JPND project ‘Dementia Outcome Measures: Charting new territory’ (2014 -2015)4.
Several funded research programmes have focussed on the theme of people with dementia themselves. These include:
- involving people with dementia in research through Patient and Public (PPI) initiatives5,6;
- capturing views of people with dementia on outcome measures7,8and terminology9;
- exploring asset-based instruments for measuring wellbeing in people with dementia10,11;
- novel methods of examining the views of people with dementia about their quality of life through use of personalised self- report instruments12.
Rationale for new methodologies
The longstanding ‘gold standard’ Randomised Control Trial (RCT) is not always the first port of call for developing evidence-based practice. RCT’s are often embedded in the UK MRC framework of 2000 (updated in 2006) 13, 14 for complex intervention research, where evidence development involves a lengthy process of 6-8 years, including assessing feasibility and acceptability, pilot testing, an RCT and implementation studies. This can delay urgently needed policies to advance dementia care practice. To accelerate uptake of innovations in dementia care, new designs and methods that involve stakeholders through all phases of the development and evaluation of complex interventions are required.
Speaking at the World Dementia Council: Global dialogue on psychosocial research in dementia 14 Bob Woods noted that while there is evidence on the efficacy of psychosocial interventions in dementia (e.g. Cochrane/ systematic reviews) that rely on RCTs, important methodological considerations are needed to advance future research 15. These include: specificity of what we are aiming for with a given intervention, differentiating between therapeutic processes-for example those requiring skilled therapists versus enhancing meaningful pleasurable activities (e.g. creative arts/ intergenerational initiatives); and understanding individual and contextual differences (i.e. what works for whom in which context and situation, why and how?); aspects associated with the context and adaptation that is required for different cultures, political systems, healthcare systems; and cultural values within various family care systems (for transcript see15 pages 21-25).
Many areas of psychosocial intervention research in dementia care and support now require methodological advancement. For example, we need:
- more tailor-made methods, focussing on the right-fit between the research question(s) and the methodology used to answer these;
- methodologies to incorporate knowledge of the processes that a practitioner/ therapist require to follow, in delivering a new intervention in their care settings;
- studies of underlying working mechanisms of a given intervention (what might work – and not work- for whom, why and how) as these relate to real-life settings, such as the context of delivery and the impact of the socio-political systems;
- methodology that embraces intervention complexity in real-world settings such as ‘spontaneous change’ and other properties that lend themselves to system- thinking approaches;
- further development of meaningful outcome measurement that are applicable to the concept of the intervention as well as the real-world of people with dementia and those that support them;
- further development of methodologies on the emerging topic of co-design and evaluation of psychosocial interventions16, undertaken together with people with dementia, caregivers, professionals, policymakers and other stakeholders.
The current UK MRC framework (updated 2021) adopts a wider scope17. It includes a ‘range of detailed guidance on specific topics and methods’ 17. The authors recommend that:
- the framework should be continually updated by taking a pluralist approach (i.e. a frame and meta-guidance), including different new research designs, approaches and methodologies in and between (or outside) the MRC phases.
- guidance is published in a web-based format, which is frequently updated with new materials, resources and case studies to enhance knowledge about new methodologies as they emerge for a given topic.
New methodological approaches are emerging, such as growing interest in realist evaluations and reviews 18, 19, theory-driven intervention development and evaluation20-22, pragmatic trials and participatory action research23-25 that are closer to the real world of the people we serve and that acknowledge the value of involving all those concerned.
Therefore there is a need for novel methodologies applied to psychosocial research in dementia care.
(For references 1-25 see Introduction and Background References).
- Moniz-Cook E, Vernooij-Dassen M, Woods R, Verhey F, Chattat R, de Vugt M, Mountain G, O’Connell M, Harrison J, Vasse E, Dröes RM, & Orrell M For the INTERDEM group. (2008) A European consensus on outcome measures for psychosocial intervention research in dementia care Aging & Mental Health 12,14- 25. doi: 10.1080/13607860801919850
- Vernooij Dassen M & Moniz-Cook E (2014) Raising the standard of applied care research: addressing the implementation error Aging & Mental Health 18, 809-14. doi: 10.1080/13607863.2014.89997
- Vasse E, Moniz-Cook E, Olde Rikkert M, Cantegreil I, Charras K, Dorenlot P, Fumero G, Franco M, Woods B, Vernooij-Dassen. M. (2012) The development of quality indicators to improve psychosocial care in dementia. Psychogeriatics 24, 921-30
- https://www.neurodegenerationresearch.eu/wp-content/uploads/2015/10/JPND-Report-Fountain.pdf
- Gove, D., Diaz-Ponce, A., Georges, J., Moniz-Cook, E., Mountain, G., Chattat, R., Øksnebjerg, L. & The European Working Group of People living with dementia (2018) Alzheimer Europe’s position on involving people living with dementia in research through PPI (Patient and Public Involvement), Aging & Mental Health, 22 (6), 723-729. doi: 10.1080/13607863.2017.1317334.
- Roberts, C., Rochford-Brennan, H., Goodrick, J., Gove, D., Diaz-Ponce, A., & Georges, J. (2020). Our reflections of patient and public involvement in research as members of the European Working Group of People with Dementia. Dementia,19, 1, 10-17. doi: 10.1177/1471301219876402.
- Øksnebjerg L., Diaz-Ponce, A., Gove, D., Moniz-Cook, E., Mountain, G., Chattat, R. and Woods, B. (2018). Towards capturing meaningful outcomes for people living with dementia in psychosocial intervention research: a pan-European consultation Health Expectations, 21, 6, 1056 – 1065 https://doi.org/10.1111/hex.12799
- Bhatt, J., Stoner, C. R., Scior, K., & Charlesworth, G. (2021). Adaptation and preliminary psychometric properties of three self-stigma outcome measures for people living with dementia. BMC Geriatrics, 21 (1), 1-12. doi: 10.1186/s12877-020-01983-0.
- Wolverson, E, Dunn, R., Moniz-Cook, E. Gove, D. and Ponce-Diaz, A. (2021). The language of behaviour changes in dementia care: the perspectives people with dementia Journal of Advanced Nursing, 77, 4, 1992-2001 https://doi.org/10.1111/jan.14787
- Clarke, C., Woods, B., Moniz-Cook, E., Mountain, G., Øksnebjerg, L., Chattat, R., Diaz, A., Gove, D., Vernooij-Dassen., M and Wolverson, E (2020) Measuring the well-being of people with dementia: a conceptual scoping review. Health & Quality of Life Outcomes,18, 249 https://doi.org/10.1186/s12955-020-01440-x
- ** Clarke, C., Baird, K., Moniz‐Cook, E., Mountain, G., Wolverson, E., Lee, E., & Hewitt, C. (2025). A psychometric study of the Flourishing Scale for people living with dementia.Alzheimer’s & Dementia: Translational Research & Clinical Interventions, 11(2), e70097 A psychometric study of the Flourishing Scale for people living with dementia – Clarke – 2025 – Alzheimer’s & Dementia: Translational Research & Clinical Interventions – Wiley Online Library
- Hendriks, I., Demetrio, R., Meiland, F.J.M., Chattat, R., Szcześniak, D., Rymaszewska, J., Evans, S.B., Atkinson, T. Farina, E., Saibene, F.L., Gerritsen, D., Dröes, R.M. (2021). Value of personalized versions of dementia-specific quality of life scales; An explorative study in three European countries Am J Alz Disease & other Dementias36,1-9. doi: 10.1177/15333175211033721.
- Medical Research Council, MRC (2000) A Framework for Development and Evaluation of RCTs for Complex Interventions to Improve Health. London: Medical Research Council
- Craig P, Dieppe P, Macintyre S, Michie S, Nazareth I, Petticrew M. (2006) Developing and Evaluating Complex Interventions. London: Medical Research Council
- Woods R. (2021): Psychosocial Interventions for People living with dementiaIn Global dialogue on psychosocial research in dementia: the dementia landscape project. World Dementia Council October2021 DLP – Transcript – Psychosocial.pdf (worlddementiacouncil.org)pp 21 -25
- Lord, K., Kelleher, D., Ogden, M., Mason, C., Rapaport, P., Burton, A., … & Cooper, C. (2022). Co-designing complex interventions with people living with dementia and their supporters. Dementia, 2, 1,426-441. doi: 10.1177/14713012211042466. Epub 2021 Dec 30.
- Skivington K, Matthews L, Simpson SA, Craig, P Baird B, Blazenby, JM, Boyd KA, Craig N, French DP, McIntosh E, Petticrew, M & Rycroft-Malone J. (2021) A new framework for developing and evaluating complex interventions: update of Medical Research Council guidance, BMJ, 374:n2061 https://doi.org/10.1136/bmj.n2061
- Duddy C. & Wong G. (2023) Grand rounds in methodology: when are realist reviews useful, and what does a ‘good’ realist review look like? BMJ Quality & Safety 32, 173-180. DOI: 10.1136/bmjqs-2022-015236
- Jack K. (2022) What is realist evaluation? Evidence-Based Nursing, 25,111-113. doi: 10.1136/ebnurs-2022-103608. Online ahead of print.
- Gilissen, J., Pivodic, L., Wendrich-van Dael, A., Gastmans, C., Vander Stichele, R., Van Humbeeck, L., … & Van den Block, L. (2019). Implementing advance care planning in routine nursing home care: the development of the theory-based ACP+ program. PLoS One, 14, 10, e0223586. doi:10.1371/journal.pone.0223586.
- Gilissen, J., Pivodic, L., Gastmans, C., Vander Stichele, R., Deliens, L., Breuer, E., & Van den Block, L. (2018). How to achieve the desired outcomes of advance care planning in nursing homes: a theory of change. BMC Geriatrics, 18,1, 1-14. DOI: 10.1186/s12877-018-0723-5
- de Nooijer, K., Pivodic, L., Van Den Noortgate, N., Pype, P., Evans, C., & Van den Block, L. (2021). Timely short-term specialized palliative care service intervention for older people with frailty and their family carers in primary care: Development and modelling of the frailty+ intervention using theory of change. Palliative Medicine, 35, 10, 1961-1974. DOI: 10.1177/02692163211040187
- Fogg, C., Lanning, E., Shoebridge, J., Longstaff, J., De Vos, R., Dawson-Taylor, K., … & Chauhan, A. (2022). The role of Participatory Action Research in developing new models of healthcare: Perspectives from participants and recommendations for ethical review and governance oversight. Ethics, Medicine and Public Health,24, 100833. https://doi.org/10.1016/j.jemep.2022.100833.
- Hsieh, C. J., Yin, P. F., Chiu, C. Y., Hsiao, Y. P., & Hsiao, Y. L. (2022). Support and Empowerment for Older Adult Spousal Caregiving of People with Mild and Moderate Dementia: A Participatory Action Research. In Healthcare,10,3,569 https://www.mdpi.com/2227-9032/10/3/569
- Harkin, D., Coates, V., & Brown, D. (2022). Exploring ways to enhance pain management for older people with dementia in acute care settings using a Participatory Action Research approach. Older People Nurs.17, e12487 https://doi.org/10.1111/opn.12487
Prevention
Leads: Associate Prof. Dr. Kay Deckers, Maastricht University, The Netherlands; Prof. Dr. Jan Steyaert, Expertise Centrum Dementie Vlaanderen, Belgium; Assistant Prof. Dr. Niels Janssen, Maastricht University
Contact Person: Kay Deckers (kay.deckers@maastrichtuniversity.nl) & Niels Janssen: niels.janssen@maastrichtuniversity.nl
Established: INTERDEM annual Meeting 2018, Barcelona, Spain
Updated: December 2025
Background
Dementia has long been considered a condition affected mainly by age and other non-modifiable factors such as gender and genetics. Over the past decades though, observational research has highlighted that the risk on dementia can significantly be reduced by addressing modifiable risk factors at the individual-level such as high blood pressure, physical inactivity, smoking, lack of challenging cognitive activities and social health, but also modifiable risk factors at the population-level such as air pollution, access to education and the broader living environment (e.g., sport facilities, green spaces).1-4 Also, these risk factors need to be addressed at middle age (40 to 75y), as dementia is a condition that develops “in slow motion”, and deterioration of the brain may be going on for up to 20 years before it results in observable cognitive decline and functional dependence.1 However, this knowledge has not been well disseminated with many people being unaware that dementia risk can be partly reduced by adopting a healthy lifestyle.5-7 Studying modifiable risk and protective factors and translation of epidemiological findings into real-world interventions to promote brain health at the individual8-10 and population11-13 level has therefore become a main research priority and included in several, though still not all, national dementia plans.14
Overall Aim:
Our aim is to share knowledge and collaborate with INTERDEM colleagues and others in identifying modifiable risk and protective factors, and in developing and implementing intervention strategies to promote a brain-healthy lifestyle to reduce dementia risk for the coming decades.
Activities 2024
- Organisation of symposia on this topic (e.g. Alzheimer Europe, IPA)
- Collaborating in research proposals (e.g. applications submitted to Horizon Europe)
- Collaborating in epidemiological projects and public health campaigns
- Organize exchanges/work visits of PhD students and staff (2 work visits completed with Bradford University (UK) & Leipzig University (Germany)
Activities 2025
- INTERDEM spotlight webinar (Presentationy by Kay Deckers, Maastricht University, NL; Jan Steyaert, Expertise Centrum Dementie Vlaanderen, Belgium; Felix Wittmann, Leipzig University, DE; Sophie Wimmers, Maastricht University NL)
- Task force meetings (online: 30/01/25; in-person (Alzheimer Europe Bologna): 06/10/25)
- Collaborating in epidemiological projects and public health campaigns
- Several TF-members involved in update of the WHO guidelines on risk reduction of cognitive decline and dementia
Activities planned for 2026
- Organize exchanges/work visits of PhD students and staff
- Support of the collaboration between INTERDEM and SPIN-D ( e.g. Active participation of task force members in the organization and with regard to various topics related to prevention such as (a) Lifestyle and health promotion interventions and (b) Biological and clinical foundations of dementia prevention)
- Reaching a consensus on the next steps in prevention—what comes after knowledge about risk reduction, risk factors, and initial campaigns? Discussion rooms planned (first meeting in January 2026)
- Task force meetings (Alzheimer Europe Dublin)
- Collaborating in research proposals (e.g., preparation of TRIPOD- application Marie Skłodowska-Curie Doctoral Training Network)
Frequency of meetings for 2026 – Three times per year (starting January 26)
References
- Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S.,…, Mukadam, N. (2020). Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. The Lancet, 396(10248), 413-446.
- World Health Organization. (2019). Guidelines on risk reduction of cognitive decline and dementia. Geneva: WHO.
- Deckers, K., van Boxtel, M. P. J., Schiepers, O. J. G., de Vugt, M., Muñoz Sánchez, J. L., Anstey, K. J.,…, Köhler, S. (2015). Target risk factors for dementia prevention: a systematic review and Delphi consensus study on the evidence from observation
- Rosenau, C., Köhler, S., Soons, L. M., Anstey, K. J., Brayne, C., Brodaty, H.,…, Deckers, K. (in press). Umbrella review and Delphi study on modifiable factors for dementia risk reduction. Alzheimer’s & Dementia.
- Heger, I., Deckers, K., van Boxtel, M., de Vugt, M., Hajema, K., Verhey, F., & Köhler, S. (2019). Dementia awareness and risk perception in middle-aged and older individuals: baseline results of the MijnBreincoach survey on the association between lifestyle and brain health. BMC Public Health, 19(1), 678.
- Kjelvik, G., Rokstad, A. M. M., Stuebs, J., Thingstad, P., Deckers, K., Köhler, S., & Selbæk, G. (2022). Public knowledge about dementia risk reduction in Norway. BMC Public Health, 22(1), 2046.
- Zülke, A. E., Luppa, M., Köhler, S., & Riedel-Heller, S. G. (2022). Knowledge of risk and protective factors for dementia in older German adults A population-based survey on risk and protective factors for dementia and internet-based brain health interventions. PLoS ONE, 17(11), e0277037.
- Heger, I., Deckers, K., de Vugt, M., Verhey, F., Oenema, A., van Boxtel, M., & Köhler, S. (2023). Using mHealth for primary prevention of dementia: A proof-of-concept study on usage patterns, appreciation, and beliefs and attitudes regarding prevention. Journal of Alzheimer’s Disease, 94(3), 935-948.
- Jones, D., Drewery, R., Windle, K., Humphrey, S., & de Paiva, A. F. (in press). Dementia prevention and the GP’s role: a qualitative interview study. British Journal of General Practice.
- Zülke, A. E., Pabst, A., Luppa, M., Roehr, S., Seidling, H., Oey, A., . . . Riedel-Heller, S. G. (2024). A multidomain intervention against cognitive decline in an at-risk-population in Germany: Results from the cluster-randomized AgeWell.de trial. Alzheimer’s & Dementia, 20(1), 615-628.
- Heger, I., Köhler, S., van Boxtel, M., de Vugt, M., Hajema, K., Verhey, F., & Deckers, K. (2020). Raising awareness for dementia risk reduction through a public health campaign: a pre-post study. BMJ Open, 10(11), e041211.
- Van Asbroeck, S., van Boxtel, M., Steyaert, J., Köhler, S., Heger, I., de Vugt, M.,…,Deckers, K. (2021). Increasing knowledge on dementia risk reduction in the general population: results of a public awareness campaign. Preventive medicine, 147, 106522.
- Paauw, D., Heger, I., Bjerre, J. F., Ringgaard, M. F., Stensgård, V., Horstkötter, D.,…,Deckers, K. (2024). Increasing awareness for dementia risk reduction through a public awareness campaign in Denmark: A pre-post study. Preventive Medicine, 179, 1.
- Steyaert, J., Deckers, K., Smits, C., Fox, C., Thyrian, R., Jeon, Y., Vernooij-Dassen, M., Köhler, S. (2021). Putting primary prevention of dementia on everybody’s agenda. Aging & Mental Health, 25(8):1376-80.
Members *
- Stephanie van Asbroeck (Maastricht University, NL)
- Tessa van Baal (Maastricht University, NL),
- Iris Blotenberg (DZNE German Center for Neurodegenerative Diseases, DE),
- Erika Borella (University of Padua, IT),
- Jeroen Bruinsma (Maastricht University, NL),
- Dympna Casey (University of Galway, IE),
- Rabih Chattat (University of Bologna, IT),
- Kay Deckers (Maastricht University, NL), Lead
- Jan DeLepeleire (KU Leuven, BE),
- Ana Diaz (Alzheimer Europe, LU),
- Priscilla Doyle (University of Galway, IE),
- Lukas Duffner (Alzheimer Europe, LU),
- Lia Fernandes (University of Porto, PT),
- Christopher Fox (University of Exeter, UK),
- Jean Georges, (Alzheimer Europe, LU),
- Veerle van Gils (Maastricht University, NL),
- Manuel Gonçalves-Pereira (University Nova Lisbon, PT)
- Rikke Gregersen (VIA University College, DK),
- Rowan Harwood (University of Nottingham, UK),
- Irene Heger (Maastricht University, NL),
- Juanita Hoe (University of West London UK),
- Eef Hogervorst (Loughborough University, UK),
- John Hudson (Lincoln University, UK)
- Ian Andrew James (Cumbria, Northumberland, Tyne and Wear NHS Foundation Trust, UK)
- Niels Janssen (Maastricht University, NL)
- Yun-Hee Jeon (University of Sydney, AUS)
- Danielle Jones (Bradford University, UK)
- Nathan de Jong (Maastricht University, NL)
- Sebastian Köhler (Maastricht University, NL)
- David Maidment (Loughborough University, UK)
- Angela Mattison (Newcastle University, UK)
- Déborah Oliveira (University Andres Bello, BR)
- Dominique Paauw (Maastricht University, NL)
- Jacqueline Parkes (University of Northampton, UK)
- Nina Possemis (Maastricht University, NL)
- Snorri Rafnsson (University of West London, UK)
- Siobhan Reilly (Bradford University, UK)
- Steffi Riedel-Heller (University of Leipzig, DE)
- Louise Robinson (Newcastle University, UK)
- Anne Marie Mork Rokstad (Norwegian Center for Ageing, NO)
- Colin Rosenau (Maastricht University, NL)
- Kritika Samsi (Kings College London, UK)
- Sharon Savage (Newcastle University, UK)
- Felicity Slocombe (Bradford University, UK)
- Justine Schneider (University of Nottingham, UK)
- Birgitte Schoenmakers (KU Leeuven, BE)
- Sarah Smith (University of Manchester, UK)
- Jens Soeterboek (Maastricht University, NL)
- Lion Soons (Maastricht University, NL)
- Jan Steyaert (Expertise Centrum Dementie Vlaanderen, BE) – co- Lead
- Rene Thyrian (DZNE German Center for Neurodegenerative Diseases, DE)
- Lotte Truin (Maastricht University, NL)
- Myrra Vernooij-Dassen (Radboud UMC Nijmegen, NL)
- Marjolein de Vugt (Maastricht University, NL)
- Karin Wolf-Ostermann (University of Bremen, DE)
- Sophie Wimmers (Maastricht University, NL)
- Karen Windle (Ulster University, UK)
- Felix Wittmann (University of Leipzig, DE)
- Andrea Zülke (University of Leipzig, DE)
* Taskforce listed in alphabetical order and includes non-INTERDEM members
Appendix
Notes taskforce prevention meeting AEC 2025 Bologna here.
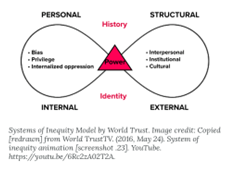
Context, culture, and intersectionality
Chairs: Prof. Dr. Martina Roes (martina.roes@dzne.de)
Co-chair: Franziska Laoporte Uribe, PhD (franziska.laporte-uribe@dzne.de)
Contact: viktoria.peters-nehrenheim@dzne.de
Established: 2019 Annual INTERDEM meeting at Alzheimer Europe, Barcelona.
Updated: May 2025
Background: This taskforce was initiated in October 2019 with the name ‘intercultural care’. Over the course of time while diving deeper into different concepts linked to ‘intercultural care’ our focus shifted toward a critical reflection of the concepts ‘context, culture, and intersectionality’. We therefore re-named our taskforce.
Context: We aim to analyze and understand how the respective constellations differ from locality to locality, from time to time, and from context to context. Understanding context means understanding both the external and internal influences on a healthcare provider, an organization or an individual. External contextual influences on an organization could be for example health policy and economics, the role of the different professions or social and ideological movements. Internal contextual influences within an organization could be linked to its culture and leadership, staff skills and personalities, or the perspective of the care recipients.
The purpose for us is to analyze context through an intersectional lens, for example to understand one’s social position which might change along the lines of citizenship (e.g. for immigrants) or using social determinants of health characteristics to understand social positioning within the dementia care context.
Culture: The use of the concept of culture (or interculture/intercultural) is very common in dementia care research. Although the term culture is often used, there is a lack of agreement on how to define it and what it encompasses. It nevertheless provides us with and awareness that different people bring a different set of experiences, expectations and predispositions to the field of dementia – whether they are people living with the disease, caregivers, health professionals or researchers. We argue, that addressing cultural can benefit from an intersectional lens, asking questions such as: who produces the text [knowledge/meaning/interpretation/discourse], what is the societal positioning of a person [within a society / the health care system / in research etc.], what were societal circumstances of the production (e.g. coming from a high-income country or writing as an indigenous person representing an ethnic minority).
The purpose for us is to understand how to engage intersectionality as a lens to conceptualize culture and societal positioning specifically of people living with dementia and their social network in different European countries and to identify way, how this changes dementia care research.
Intersectionality: Intersectionality comes from the work of black feminist scholars and activists. Intersectionality is a theoretical framework rooted in the premise that human experience is jointly shaped by multiple societal positioning (e.g. ethnicity, gender, age), social health determinants, and cannot be adequately understood by considering social positions independently.
 Examples for personal characteristics [1]
Examples for personal characteristics [1]
Intersectionality argues that personal identities such as gender, age, education, ethnicity, sexuality, and other markers of difference intersect and reflect large social structures of power, oppression and privilege.
Intersectionality argues that large power structures intersect and lead to oppression and privilege based on our social positioning such as ethnicity, age, gender, education, sexuality within the society we live in.
The purpose for us is to critically reflect on the framework of intersectionality, its translational potential and ways of applying it to dementia care research.
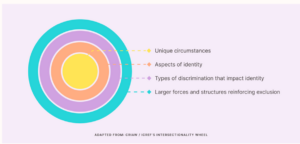 [2]
[2]
Overall aims
This INTERDEM Taskforce ‘context, culture, and intersectionality’ aims to bring an intersectional lens to dementia care and research with the aim of going beyond understanding inequality and inequity. Our overall understanding is, that we need to move away from the assumption that everyone is positioned equally, which means that we need to start thinking from the perspective of difference. We need to acknowledge, that we are all (1) different in the first place and therefore (2) need to understand why existing theories, interventions, health frameworks don’t work for everyone, and (3) how to overcome these conceptual gaps. Therefore, the often-used research outcome of ‘generalization’ will be challenged and we will need to understand how to conduct research differently and still meet overarching requirements in research.
This is linked to the objective to improve the understanding of the mechanism of health (care) disparities and how these affect the amount and quality of care received by a person with dementia and their significant others / social network. Intersectional approaches help to identify inequity and inequality within and between groups of people but also within health care systems based on the way multiple facets of an individual’s identity interact. An intersectional approach also helps to examine data practices, processes, and institutions reflectively.
It’s in our interest to ‘use’ intersectionality as an analytical framework or analytical sensibility, in some ways resulting in us ‘doing’ it.
Objectives of the taskforce
We aim to embed innovative methodologies into dementia care research, specifically intersectionality as a research topic and applying an intersectional lens in dementia care research. More methodological work is required to (1) ensure researchers understand key features that define intersectionality analyses, (2) improve reporting practices for intersectional analyses, and (3) develop and adapt designs and methods.
Additionally, there is a need to better understand how to engage and involve underrepresented population groups (often excluded based on their personal characteristics) in research, either as co-researchers or as study participants.
Moreover, we need to develop an idea of how to use an intersectional lens when conducting reviews, since intersectionality refers to the examination of how multiple social identities intersect to shape the production and interpretation of texts within various contexts.
As a taskforce we aim to provide high quality training and consultancy to other INTERDEM taskforces as well as collaborate across taskforces, members and particularly to the INTERDEM academy as we are committed to equity not just for people living with dementia and families but our researchers too. We feel strongly about intersectionality cutting across psychosocial dementia research and therefore will be committed to helping peers and colleagues in achieving and applying and intersectional lens.
References (used above)
- Bauer GR, et al. (2021) Intersectionality in quantitative research: A systematic review of its emergence and applications of theory and methods. SSM Popul Health. 2021 Apr 16;14:100798. doi: 10.1016/j.ssmph.2021.100798. PMID: 33997247; PMCID: PMC8095182.
- Kelly, C., et al. (2021) ‘Doing’ or ‘using’ intersectionality? Opportunities and challenges in incorporating intersectionality into knowledge translation theory and practice. Int J Equity Health 20, 187 (2021). https://doi.org/10.1186/s12939-021-01509-z
- Roes et al. (2022) Intersectionality and its relevance for research in dementia care of people with a migration background. Z Gerontol Geriatr. 2022 Jul;55(4):287-291. English. doi: 10.1007/s00391-022-02058-y. Epub 2022 Apr 7. PMID: 35391538.
Ongoing Activities 2024/2025
- Involved in the JPND founded project
Innovations in diversity and Equity in Social health research in Dementia [INTEREST] Project lead Prof. Louise Hopper (Dublin City University, Ireland)
(please add website link on interdem website)- Neal D. et al. (2025) Effective for Whom? A Review of Psychological and Social Intervention Recommendations in European Dementia Care Guidelines Through the Lenses of Social Health and Intersectionality. Sci.2025, 15, 457. https://doi.org/10.3390/bs15040457
- Wei, Roes et al. (under review) What are the unmet needs in people affected by dementia? A scoping review of reviews.
- Roes & Wei et al. (in preparation) Secondary analysis of the scoping review of unmet needs through an intersectional analysis
- Involved in discussing health disparities with the European Working Group of People with Dementia (EWGPWD) as well as the Caregiver Dementia Working Group (EDCWG) (both embedded in Alzheimer Europe)
- Frontiers Dementia:
Co-Editor of a Special issue on Diversity and inclusive practices in dementia care
see call: https://www.frontiersin.org/research-topics/66900/diversity-intersectionality-and-inclusive-practices-in-dementia-care-and-research- Roes M et al. [Taskforce Action] [in preparation] Scoping review on intersectionality research in dementia care
- Kuebra Altinol (Interdem Academy) (under review) Intersectional research in dementia care of people from culturally and linguistically diverse background: A scoping review
- Wei, Roes et al. (under review) What are the unmet needs in people affected by dementia? A scoping review of reviews.
- Roes & Wei et al. [in preparation] Secondary analysis of the scoping review of unmet needs through an intersectional analysis
- Developing a Guideline for Inclusive Dementia Research (G-IDR)
Project Lead Carolien Smits at Pharos, Netherland - Across Taskforce Special Interest Group (SIG) on Relationships, Intimacy, and Sexuality [contact person: Hannah Christie]
- Position paper on stigmatization [contact person: Golnaz Atefi]
- Future research priorities for gender-sensitive non-pharmacologic therapies to reduce dementia-related symptoms in people with dementia – using Priority Setting Partnership [GenderDem] (starts in July 2025)
Project Lead Prof. Martina Roes
Dissemination since 2021
- Alzheimer Europe Conference [Oct. 2025, Bologna]
Interdem Symposium on the JPND funded project INTEREST - Alzheimer Europe Conference [Oct. 2025, Bologna]
plenary session Topic: Gender and sexuality in dementia
Topic: Understanding the needs, wishes, and experiences of lesbian, gay, bisexual or transgender (LGBT) people - Alzheimer Europe Conference [Oct 2024, Geneva] Topic: Interdem Symposium together with the taskforce Inequalities in Dementia Care
- Nursing Science Symposium Improving research impact for families experiencing acute-critical illness at the Institute for Implementation Science in Health Care (IfIS), University of Zurich [Jan 2024] Topic: Complex implementation challenges – addressing implementation Equity
- Interdem Academy Spotlight [Dec 2023, virtual] Topic: How can dementia care research benefit from an intersectional lens?
- FPO Conference at UK Hull University [June 2022, virtual] Topic: Lens of Intersectionality – What is it all about?
- GSA Conference [Nov. 2022, Indianapolis] Topic: Building and Leveraging Community Partnership that Embrace Diversity, Enrich Discovery, and Reimagine Aging (Presidential Symposium)
- Alzheimer Europe Conference [Oct. 2022, Bucharest]:
Topic: Interdem Symposium Applying an intersectional lens in dementia care research - Social Health Taskforce [May 2021, virtual] Topic: Social Heath through the Lens of Intersectionality
Frequency of meetings
Virtual meetings every month: either the large group or smaller groups, with a focus on a specific task such as conducting the scoping review, discussing progress of INTEREST)
Additionally, in-person meetings at the annual AE conference.
Meetings in 2025: 10-11.30 am (German time) virtually
21st Feb., 21st March, 23rd May, 20th June, 18th July, 15th Aug., 19th Sept, 17th Oct., 21st Nov, 19th Dec
Members (alphabetical order)
| Name | Institution | Interdem | Position | ||
| 1 | Kuebra Altinok | DZNE Witten, Germany | kuebra.altinok@dzne.de | Academy | junior |
| 2 | Golnaz Atefi | Maastricht University, NL | g.atefi@maastrichtuniversity.nl | Academy | postDoc |
| 3 | Saloua Berdai | Vrije University, Belgium | saloua.berdai@kdg.be | postDoc | |
| 4 | Jem Bhatt | University of London, United Kingdom | jemini.bhatt.15@ucl.ac.uk; jemini.bhatt@ucl.ac.uk | Academy | PostDoc |
| 5 | Georgina Charlesworth | University of London, United Kingdom | g.charlesworth@ucl.ac.uk | yes | Professor |
| 6 | Rabih Chattat | University of Bologna, Italy | rabih.chattat@unibo.it | yes | Professor Interdem Board |
| 7 | Ann Claey | Vrije University, Belgium | Ann.claeys@vub.be | junior | |
| 8 | Hannah Christie | Maastricht University, NL | hannah.christie @maastrichtuniversity.nl |
Academy | PostDoc |
| 9 | Simone Anna Felding | DZNE Witten, Germany | SimoneAnna.Felding@dzne.de | Academy | Junior |
| 10 | Clarissa Giebel | University of Liverpool, U.K. | Clarissa.Giebel@liverpool.ac.uk | yes | postDoc |
| 11 | Silvia Gonella | Department of Public Health and Pediatrics, University of Torino | silvia.gonella@unito.it | Assistant Professor of Nursing (RUTD – B) | |
| 12 | Diane Gove | Alzheimer Europe, Luxembourg | dianne.gove@alzheimer-europe.org | yes | PostDoc |
| 13 | Catrin Jones | University of Worchester, United Kingdom | Catrin.jones@worc.ac.uk | yes | postDoc |
| 14 | Franziska Laporte Uribe | DZNE Witten, Germany | Franziska.Laporte-Uribe@dzne.de | yes | postDoc |
| 15 | Nicole Mueller | University College Cork, Ireland | nicole.muller@ucc.ie | yes | Professor |
| 16 | Shadreck Mwale | Geller Institute of Ageing and Memory, School of Biomedical Sciences, University of West London | Shadreck.Mwale@uwl.ac.uk | yes | Associate Professor |
| 17 | Sahdia Parveen | Bradford University, United Kingdom | S.Parveen27@bradford.ac.uk | yes | Associate Professor |
| 18 | Martina Roes | DZNE Witten, Germany | Martina.Roes@dzne.de | yes | Chair, Interdem Board |
| 19 | Kritika Samsi | King’s College London, U.K. | kritika.1.samsi@kcl.ac.uk | yes | PostDoc |
| 20 | Carolien Smits | Pharos, NL | C.Smits@pharos.nl | yes | Co-Chair |
| 21 | Huerrem Tezcan | Alice Salomon University of Applied Science, Germany | tezcan@ash-berlin.eu | Professor | |
| 22 | René Thyrian | DZNE Greifswald, Germany | Rene.Thyrian@dzne.de | yes | Professor |
| 23 | Chantal Van Audenhove | KU Leuven, Belgium | chantal.vanaudenhove@kuleuven.be | yes | PostDoc |
| 24 | Bryony Waters | Nottingham University, U.K. | Bryony.Waters1@nottingham.ac.uk | Academy | Junior |
| Retired | |||||
| Aud Johannessen:
|
University of South-Eastern Norway USN, Norway | Aud.Johannessen@usn.no | Yes / retired | Retired Professor | |
Publications by members as 1st or co-authors (corresponding articles)
Annac K, Basyigit M, Öztürk S, Örs ER, Aksakal T, Kuhn C, Rutenkröger A, Tezcan-Güntekin H, Yilmaz-Aslan Y, Brzoska P. Diversity-On: A Diversity-Sensitive Online Self-Help Program for Family Caregivers-A Protocol for a Mixed Methods Study. J Adv Nurs. 2024 Sep 10. doi: 10.1111/jan.16443. Epub ahead of print. PMID: 39253765.
Berdai Chaouni S, Claeys A, van den Broeke J, De Donder L. Doing research on the intersection of ethnicity and old age: Key insights from decolonial frameworks. J Aging Stud. 2021 Mar;56:100909. doi: 10.1016/j.jaging.2020.100909. Epub 2020 Nov 28. PMID: 33712097.
Berdai Chaouni S, Smetcoren AS, De Donder L. Caring for migrant older Moroccans with dementia in Belgium as a complex and dynamic transnational network of informal and professional care: A qualitative study. Int J Nurs Stud. 2020 Jan;101:103413. doi: 10.1016/j.ijnurstu.2019.103413. Epub 2019 Sep 6. PMID: 31678839.
Bhatt, J. (2024). The influence of stigma on disclosure decision-making in people affected by dementia (Doctoral dissertation, UCL (University College London).
Bhatt J, Kohl G, Scior K, Charlesworth G, Muller M, Dröes R-M. Comparing the stigma experiences and comfort with disclosure in Dutch and English populations of people living with dementia. Dementia. 2023;22(7):1567-1585. doi:10.1177/14713012231188503
Bhatt J, Scior K, Stoner CR, Moniz-Cook E, Charlesworth G. Stigma among UK family carers of people living with dementia. BJPsych Open. 2022 Oct 7;8(6):e179. doi: 10.1192/bjo.2022.585. PMID: 36205002; PMCID: PMC9634559.
Bhatt J, Stoner CR, Scior K, Charlesworth G. Adaptation and preliminary psychometric properties of three self-stigma outcome measures for people living with dementia. BMC Geriatr. 2021 Jan 9;21(1):34. doi: 10.1186/s12877-020-01983-0. PMID: 33422016; PMCID: PMC7796608.
Bosma C, Smits C. Behavioral changes in migrants with dementia: Experiences of professional caregivers. Z Gerontol Geriatr. 2022 Jul;55(4):281-286. English. doi: 10.1007/s00391-022-02057-z. Epub 2022 Apr 8. PMID: 35394191.
Cheston R, Dodd E, Smith P, Woodstoke NS, Jutlla K, Fry G, Truswell D, Butt J, Parveen S. “You just can’t do that in dementia care”: Barriers to partnership working within dementia services for people from south Asian communities. Dementia (London). 2024 Sep 14:14713012241283189. doi: 10.1177/14713012241283189. Epub ahead of print. PMID: 39277785.
Giebel C, Harding E, Volkmer A, Chirico I, Hopper L, Szczesniak D, Talbot CV, Diaz-Ponce A, Gove D, Knapp M, Robinson L, Rahman-Amin M, Thyrian R, Hanna K; INTERDEM Taskforce on Inequalities in Dementia Care. The landscape of inequalities in dementia across Europe: First insights from the INTERDEM taskforce. Int J Geriatr Psychiatry. 2024 May;39(5):e6096. doi: 10.1002/gps.6096. PMID: 38719786.
Giebel C (2024) A new model to understand the complexity of inequalities in dementia. Int J Equity Health 23, 160 (2024). https://doi.org/10.1186/s12939-024-02245-w
Gove D, Nielsen TR, Smits C, Plejert C, Rauf MA, Parveen S, Jaakson S, Golan-Shemesh D, Lahav D, Kaur R, Herz MK, Monsees J, Thyrian JR, Georges J. The challenges of achieving timely diagnosis and culturally appropriate care of people with dementia from minority ethnic groups in Europe. Int J Geriatr Psychiatry. 2021 Dec;36(12):1823-1828. doi: 10.1002/gps.5614. Epub 2021 Aug 18. PMID: 34378237; PMCID: PMC9291493.
Kohl, G., Ulate, M.M., Bhatt, J., Scior, K. and Charlesworth, G. (2021), Factors associated with disclosing a diagnosis of dementia to one’s social network: A systematic review. Alzheimer’s Dement., 17: e056146. https://doi.org/10.1002/alz.056146
Neal D., Bartels S., Berdai Chaouni S., […] Giebel C. […], Roes M. et al. (2025) Effective for Whom? A Review of Psychological and Social Intervention Recommendations in European Dementia Care Guidelines Through the Lenses of Social Health and Intersectionality. Behav. Sci. 2025, 15, 457. https://doi.org/10.3390/bs15040457
Peters-Nehrenheim V, Rommerskirch-Manietta M, Purwins D, Roes M, Tezcan-Güntekin H. Care preferences of older migrants and minority ethnic groups with various care needs: a protocol for a scoping review. BMJ Open. 2022 Nov 21;12(11):e061712. doi: 10.1136/bmjopen-2022-061712. PMID: 36410833; PMCID: PMC9680146.
Roes M, Laporte Uribe F, Peters-Nehrenheim V, Smits C, Johannessen A, Charlesworth G, Parveen S, Mueller N, Hedd Jones C, Thyrian R, Monsees J, Tezcan-Güntekin H. Intersectionality and its relevance for research in dementia care of people with a migration background. Z Gerontol Geriatr. 2022 Jul;55(4):287-291. English. doi: 10.1007/s00391-022-02058-y. Epub 2022 Apr 7. PMID: 35391538.
Shafiq S, Parveen S, Oyebode JR. How people of African Caribbean or Irish ethnicity cope with long-term health conditions in UK community settings: A systematic review of qualitative, quantitative and mixed method studies. Health Soc Care Community. 2021 Mar;29(2):319-327. doi: 10.1111/hsc.13181. Epub 2020 Oct 7. PMID: 33025714.
Tezcan-Güntekin H, Özer-Erdogdu I, Yilmaz-Aslan Y, Aksakal T, Bird R. Ethical and Methodological Challenges in Research With Hard-to-Reach Groups: Examples From Research on Family Caregivers for Migrant Older Adults Living With Dementia. Gerontologist. 2022 Jul 15;62(6):823-831. doi: 10.1093/geront/gnab179. PMID: 34875066; PMCID: PMC9290906.
Yilmaz-Aslan Y, Annac K, Aksakal T, Yilmaz H, Merz S, Wahidie D, Razum O, Brzoska P, Tezcan-Güntekin H. What Self-Management Skills Do Turkish Caregivers Have in Caring for People with Dementia? Results of a Qualitative Survey. Healthcare (Basel). 2024 Jun 12;12(12):1187. doi: 10.3390/healthcare12121187. PMID: 38921301; PMCID: PMC11202945.
[1] https://researchguides.library.syr.edu/fys101/intersectionality
[2] https://www.cultureamp.com/blog/why-intersectionality-matters
Inequalities in Dementia Care
Leads: Dr Clarissa Giebel & Dr Kerry Hanna, University of Liverpool, UK
Contact: Clarissa.Giebel@liverpool.ac.uk
Established: 2022, Alzheimer’s Europe Conference in Bucharest
Updated: October 2025
Background
People with dementia and their carers are facing various barriers in accessing and using good quality post-diagnostic dementia care. These include socio-economic background characteristics, living situation and area, cultural background, dementia subtype, and health and digital illiteracy. In addition, there are systemic barriers to accessing care, including how systems are funded, decision making processes about who receives financial support to access care, and training and support of the care workforce. Within the community, there can also be additional barriers.
Aims:
Considering the different health and care infrastructures and population characteristics across Europe, we want to understand and learn from differences across countries. Outputs from this workforce can inform policy and decision-making.
The aims of this Taskforce are to:
- explore and address system-, community-, and individual-level inequalities in providing, accessing and using post-diagnostic dementia care across Europe;
- generate learnings for other countries where certain inequalities (incl. socio-economic, literacy, health infrastructure, digital) may be more pronounced than in others; and
- provide cross-country best practice/system recommendations for enabling more equitable dementia care across Europe.
This Taskforce aspires to have strong public, charity, non-academic stakeholder involvement. We will ensure that people with dementia and unpaid carers contribute to activities.
Activities:
- Position paper (with definitions) on system, community and individual level inequalities across Europe, and what this Taskforce sets out to achieve (published November 2023)1
- A systematic review into misdiagnosis of dementia (published October 2024) 2
- A systematic review on solutions to inequalities in dementia diagnosis/care (Timeline: PROSPERO registered: CRD42024504882, completed late October)
- Systematic review on European data sets capturing His in dementia (in progress)
- Online survey about awareness of dementia subtypes (in progress)
Activities for 2026:
- Continue with quarterly virtual team meetings (members interested in presenting their HI work contact CG)
- Complete activities (3)-(5) & British Academy Study
- Identify further international grant funding opportunities
Funding:
- 1st joint grant application: COST Centre application (to be resubmitted)
- 1st funded study: Britisch Academy „Disconnect between H&SC in dementia“
- 2nd joint grant application. Horizon Europe (under review)
Meeting frequency: bimonthly – see Appendix for dates
References
- Giebel C, Harding H, Volkmer A, Chirico I, Hopper L, Szczesniak D, et al. The landscape of inequalities in dementia across Europe: First insights from the INTERDEM Taskforce. Int J Geriatr Psychiatry. 2024; e6096. https://doi.org/10.1002/gps.6096
- Giebel C, Silva-Ribeiro W, Watson J, Volkmer A, Chirico I, Diaz A, Heath B, Hanna K, Talbot C. A Systematic Review on the Evidence of Misdiagnosis in Dementia and Its Impact on Accessing Dementia Care. Int J Geriatr Psychiatry. 2024; 39: e6158. https://doi.org/10.1002/gps.6158
Task Force Members *
- Sara Bartels (Maastricht University, Netherlands/Karolinska Institute, Sweden);
- Dympna Casey (University of Galway, Ireland);
- Georgina Charlesworth (University College London, UK);
- Rabih Chattat (University of Bologna, Italy);
- Ilaria Chirico (University of Bologna, Italy);
- Adelina Comas-Herrera (LSE, UK);
- Ana Diaz (Alzheimer’s Europe, Luxembourg);
- Simone Fielding (DZNE, Germany);
- Debbie Gerritzen (Radboud University, Netherlands);
- Clarissa Giebel (University of Liverpool, UK) – lead;
- Diane Gove (Alzheimer’s Europe; Luxembourg);
- Kerry Hanna (University of Liverpool, UK) – co-lead;
- Bronte Heath (Alzheimer Society, UK);
- Catrin Hedd Jones (Bangor, UK);
- Eithne Heffernan (University of Nottingham, UK);
- Louise Hopper (Dublin City University, Ireland);
- Karan Jutlla (Wolverhampton, UK);
- Sebastian Köhler (Maastricht University, Netherlands);
- Martin Knapp (LSE, UK);
- Fritze Kristensen (Aarhuis University, Denmark);
- Orii McDermott (Nottingham, UK);
- Naaheed Mukadam (UCL, UK)
- Malayka Rahman-Amin (Alzheimer Society, UK);
- Martina Roes (DZNE, Germany);
- Miguel Padeiro (University of Coimbra, Portugal);
- Louise Robinson (Newcastle University, UK);
- Kritika Samsi (Kings College London, UK);
- Duygu Sezgin (University of Galway, Ireland);
- Carolien Smits (Pharos, Netherlands);
- Jan Steyart (University of Antwerp Belgium);
- Dorota Szcezniak (Wroclaw University, Poland);
- Marjorlein Thijssen (Radboud University, Netherlands);
- Rene Thyrian (DZNE, Germany);
- Hilde Verbeek (Maastricht University, Netherlands);
- James Watson (University of Liverpool, UK);
- Gill Windle (Bangor, UK);
- Karin Wolf-Osterman (Germany).
* Taskforce listed in alphabetical order and includes non-INTERDEM members
Appendix
Meeting Dates
8th December 2022: 3rd February 2023; 28th April 2023; 16th June 2023; 4th September 2023; AE Conference – Helsinki (October 2023); 1st November 2023; 30th January 2024; 25th March 2024
Dementia Education and Training
Lead (s): Professor Jacqueline Parkes, University of Northampton (UK); Co- lead Dr Leah Macaden, University of Edinburgh, (Scotland, UK)
Contact: Jacqueline.parkes@northampton.ac.uk
Established: May 2025
Background/Rationale:
The new INTERDEM Education and Training Taskforce will play a key role in identifying, researching/evaluating, and developing evidence-based education and training for health and social care professionals, students and researchers which focuses on psychosocial care and support (including psychosocial interventions) throughout the dementia journey across Europe and beyond. The Taskforce will collaboratively engage with all INTERDEM Taskforces to identify contemporary knowledge and scientific evidence which will underpin the creation of a European Dementia Education and Training Framework.
Problem to be addressed:
In some European countries, National Knowledge and Skills Frameworks in the field of Dementia Support and Care have been in existence for at least a decade; however, there is limited research which evaluates the widespread application or impact of these frameworks in improving the knowledge and skills of health and social care professionals who support people living with dementia and their family. Some European countries may already be in the process of developing their local, regional, and national dementia education and training standards and frameworks or have not yet embarked on such a task and may feel they could benefit from an opportunity to share knowledge and understanding in relation to the design and delivery of education and training that enhances the knowledge and skills of professionals, students, and researchers working in this field. Currently, there does not appear to be a European-wide Knowledge, competencies, and Skills Framework, with associated training standards, which could underpin the effective psychosocial care and support of individuals impacted by cognitive decline. Ultimately, this Taskforce will identify new educational opportunities across Europe and beyond (where relevant); design research to identify the key components of courses/ programmes i.e. the content to be included, and the effective andragogical/ pedagogical approaches to delivery i.e. how training should be delivered. Ultimately, the aim will be to develop some guiding education/training principles and strategies, which could underpin a European Knowledge and Skills Framework and Standards; and disseminate the findings and recommendations.
Aims: The purpose of this Dementia Education and Training Task Force will be to:
- Exchange good practices from our respective countries regarding dementia care and support education (WHAT students/researchers/health and social care professionals learn and develop; HOW students/researchers/care professionals learn and develop).
- Collaboratively engage with other INTERDEM Taskforces to identify and develop contemporary research evidence which can underpin dementia education and training for students/researchers/health and social care professionals.
- Develop guiding principles and strategies for the design, development, evaluation and implementation of education and training for health and social care professionals, students, and researchers who work, or will work, on the frontline with people living with dementia and their families, as part of the future workforce.
- Identify the factors influencing the uptake of education and training for students, researchers, and health and social care professionals working in different settings.
Activities:
Phase 1: Development Tasks
- Broaden the expertise and knowledge-base of the Taskforce Team to include more representation from the INTERDEM area e.g. Including more East EU countries and diverse population groups.
- Establish effective collaborations with INTERDEM Taskforces to work on specific dementia-related education and training needs and requirements i.e. younger onset dementia etc.
- Undertake a review of dementia education and training opportunities (modules, courses, programmes, and bespoke Continuing Professional Development days) which focus on timely psychosocial care and support throughout the dementia journey for both the person living with dementia and their families. These opportunities can be for health and social care professionals and students.
- Collaborate with expert-by-experience groups (including people with dementia eg. The EWGPWD, family caregivers, health and social care professionals, educators, students and researchers) to provide a perspective on the format and content of training for health and social care professionals in delivering effective and timely psychosocial support throughout the dementia journey.
- Compare the content, delivery format, level of assessment, and skills development to share knowledge, competencies and learning, from example of good practices with the aim of developing a common core framework, while celebrating and respecting country-specific aspects.
Phase 2: Implementation Tasks
- Identify key factors (facilitators and barriers) to the uptake of training and education of health and social care professionals working in different settings across Europe.
- The development of a future output for example, a European wide Framework, or set of guiding principles.
- Disseminate the findings and recommendations of the education and training inventory and evaluative comparisons via Taskforce Meetings, Journal Publications, Conference Events, and the Annual Alzheimer Europe INTERDEM meeting.
Planned Frequency of meetings: initially monthly via an online platform
References:
- NHS England, Dementia Training Standards Framework, https://www.skillsforhealth.org.uk/resources/dementia-2015-updated-2018/
- Social Care Wales (2016) Good Work Dementia Learning and Development Framework for Wales
- https://socialcare.wales/cms-assets/documents/Good-Work-Dementia-Learning-And-Development-Framework.pdf
- Ottoboni, G., Chirico, I., Povolná, P., Dostálová, V., Holmerová, I., Janssen, N., Dassen, F., de Vugt, M., Sánchez-Gómez, Ma. C., García-Peñalvo, F., Franco-Martin, M. A., & Chattat, R. (2021). Psychosocial care in dementia in European higher education: Evidence from the SiDECar (“Skills in DEmentia Care”) project. Nurse Education Today, 103, 104977. https://doi.org/10.1016/j.nedt.2021.104977
- Surr, C. A., Gates, C., Irving, D., Oyebode, J., Smith, S. J., Parveen, S., Drury, M., & Dennison, A. (2017). Effective Dementia Education and Training for the Health and Social Care Workforce: A Systematic Review of the Literature. Review of Educational Research, 87(5), 966-1002. https://doi.org/10.3102/0034654317723305
- The Higher Education for Dementia Network (HEDN) https://hedn50472928.wordpress.com/five-year-strategy-2022-2027/ Dementia UK and HEDN [including Knifton, C. as one of the author contributors] (2013) Curriculum for Dementia Education.
Task Force members
- Dr. Jacqueline Parkes, Professor of Applied Mental Health, UON Dementia Research and Innovation Centre, University of Northampton. UK
- Ir. Leonie Copraij Windesheim University of Applied Sciences, Division Health and Social Care, Research Group Living Well with Dementia, the Netherlands
- Dr. Stephanie Daley, Reader in Mental Health & Dementia, Brighton and Sussex Medical School. UK
- Dr. ir. Simone de Bruin, Professor Living well with Dementia, Windesheim University of Applied Sciences, Division Health and Social Care, Research Group Living Well with Dementia, the Netherlands
- Dr. Ulla Eloniemi-Sulkava, Institute of Public Health and Clinical Nutrition, University of Eastern Finland, Finland
- Yvonne Feeney, PhD student, University: Brighton and Sussex Medical School, UK
- Dr. Catrin Hedd Jones, Dementia studies lecturer, Dementia Services Development Centre (DSDC), Bangor University, Wales. UK
- Dr Chris Knifton, Associate Professor in Teaching and Learning (Neurocognitive and Neurodevelopment care), De Montfort University, UK
- Dr. Leah Macaden, Senior Lecturer in Nursing Studies, School of Health in Social Science, University of Edinburgh, Scotland.
- Dr. Franka Meiland, Amsterdam University Medical Center, Department of Medicine for Older people, the Netherlands.
- Dr. Martina Roes, Professor for Nursing Science and Health Care Research, University of Witten/Herdecke, Faculty of Health, Department of Nursing Science, Witten, Germany
- Dr Anthony Scerri, Senior Lecturer, Department of Nursing/Faculty of Health Sciences,
- University of Malta.
- Dr. Charles Scerri, Professor of Dementia Studies, Department of Pathology, Faculty of Medicine & Surgery, University of Malta.
- Dr. Carolien Smits, Department of Older Adults and HealthPharos, the Netherlands
- Dr. Ozgul Uysal, Assistant Professor, Erasmus School of Social and Behavioural Sciences, the Netherlands.
- Dr Alison Ward, Deputy Director Research, Evidence and Impact, Dementia UK
Publications: [The publications provided by team members reflect the skills and strengths within the team and therefore include papers on dementia research, education and training and public involvement and engagement].
- Attard, R., Sammut, R., & Scerri, A. (2020). Exploring the knowledge, attitudes and perceived learning needs of formal carers of people with dementia. Nursing older people, 32(3).
- Banerjee S, Jones C, Wright J, Grosvenor W, Hebditch M, Feeney Y, Mackrell S, Nilforooshan R, Fox C, Bremner S, Daley S, 2021, A comparative study of the effect of the Time for Dementia programme on medical students, International Journal of Geriatric Psychiatry, http://doi.org/10.1002/gps.5532
- Banerjee S, Farina N, Daley S, Grosvenor W, Hughes L, Hebditch M, Mackrell S, Niforooshan R, Wyatt C, de Vries K, Haq I, Wright J, 2017, How do we enhance undergraduate healthcare education in dementia? A review of the role of innovative approaches and development of the Time for Dementia programme, International Journal of Geriatric Psychiatry, 32, 68-75
- Bickford B, Daley S, Sleater G, Hebditch M, Banerjee, S, 2019, Understanding compassion for people with dementia in medical and nursing students, BMC Medical Education, 19:35 https://doi.org/10.1186/s12909-019-1460-y
- Cashin, Z, Daley S, Hebditch M, Hughes L, Banerjee S, 2019, Involving people with dementia and their carers in dementia education for undergraduate healthcare professionals: a qualitative study of motivation to participate and experience, International Psychogeriatrics, 31, 6, 869-876 http://dx.doi.org/10.1017/S1041610218001357
- Chamberlain, l., Anderson, C., Knifton, C., and Madden G (2018) Suicide risk in informal carers of people living with dementia. Nursing Older People. RCNi
- Daley S, Hebditch M, Feeney Y, Pooley J, Pietersen H, Banerjee S, 2023, Understanding the experiences of people with dementia and their family carers participating in healthcare student dementia education: a mixed-methods evaluation from the Time for Dementia programme, Dementia, ttps://journals.sagepub.com/doi/epub/10.1177/14713012231191412
- Daley, S, Hebditch, M, Jones, C, Bremner S, Feeney Y, Towson G, Wright J, Banerjee S, 2023, Time for Dementia: quantitative evaluation of a dementia education programme for healthcare students, International Journal of Geriatric Psychiatry, e5922. https://doi.org/10.1002/gps.5922
- Daley S, Feeney Y, Grosvenor W, Hebditch M, Morley L, Sleater G, Wright J, Banerjee S,2020, A qualitative evaluation of the effect of a longitudinal dementia education programme on healthcare student knowledge and attitudes, Age and Ageing, 1–7 doi:10.1093/ageing/afaa182
- Feeney F, Daley S, Flaherty, Banerjee, 2021, Barriers and facilitators to implementing a longitudinal dementia education programme into undergraduate healthcare curricula: a qualitative study, BMC Medical Education, https://doi.org/10.1186/s12909-021-02632-9
- Jones, C.H., Seddon, D., Algar-Skaife, K., Maddock, C. and Green, S. (2023), “Involving older adults and unpaid carers in the research cycle: reflections on implementing the UK national standards for public involvement into practice”, Quality in Ageing and Older Adults, https://www.emerald.com/insight/content/doi/10.1108/QAOA-03-2023-0019/full/html
- Knifton, C., and Yates, S. (2019) A history of problematizations for dementia education. Journal of Nursing Research, ¾: 212-230.
- Knifton, C. et al (2019) Dementia education in HEIs: now and in the future. Journal of nursing Reserach. 24: 271-278.
- Knifton, C. (2019) Learning disability and dementia. In, Harison dening, K (ed) Evidence –based practice in dementia for nurses and nursing students. London: Jessica kingsley.
- Knifton, C (2018) Teaching students to be knowledgeable and compassionate about dementia care. Nursing Older People, RCNi
- Knifton, C., et al (2014) Making a difference in dementia education: developing a consistent and inclusive approach for all. Journal of Dementia Care, 22(4): 18-21
- Lyons, V., Oliver, E., Knifton, C., and Molesworth S. (2022) Role of Admiral nurses in supporting people with learning disabilities and dementia. Learning disability Practice [online] 12/05/22
- Macaden L (2016). Being Dementia Smart (BDS): A Dementia Nurse Education Journey in Scotland. International Journal of Nursing Education Scholarship. Volume 13, Issue 1, ISSN (Online) 1548-923X, ISSN (Print) 2194-5772, DOI: 10.1515/ijnes-2015-0019.
- Macaden L, Muirhead K (2024). Dementia Education for Workforce Excellence: Evaluation of a Novel Bichronous Approach. Healthcare. 12(5):590. https://doi.org/10.3390/healthcare12050590
- Muirhead K, Macaden L, Smyth K, Chandler C, Clarke CL, Polson R, O’Malley CT (2022). The Characteristics of Effective Technology-enabled Dementia Education: A Systematic Review and Mixed Research Synthesis https://doi.org/10.1186/s13643-021-01866-4
- Muirhead K, Macaden L, Smyth K, Chandler C, Clarke CL, Polson R, O’Malley CT (2021). Establishing the Effectiveness of Technology-enabled Dementia Education for Health and Social Care Practitioners. https://doi.org/10.1186/s13643-021-01781-8.
- Muirhead K, Macaden L, Clarke CL, Smyth K, Polson R, O’Malley CT (2019). The characteristics of effective technology enabled dementia education for health and social care practitioners: Protocol for a mixed studies systematic review. Systematic Reviews, https://doi.org/10.1186/s13643-019-1212-4
- Parkes, J.H, Pyer, M., Wray, P. and Taylor, J. (2014) Partners in projects: preparing for public involvement in health and social care research. Health Policy. 117(3), pp. 399-408. 0168-8510.
- Parkes, J., O’Malley, M., Stamou, V., La Fontaine, J., Oyebode, J.R., Carter, J. (2022) Lessons learnt from delivering the public and patient involvement forums within a younger onset dementia project. Dementia; the international journal of social research and practice, 21 (7), pp. 1-14.
- Scerri, A., & Scerri, C. (2013). Nursing students’ knowledge and attitudes towards dementia—a questionnaire survey. Nurse Education Today, 33(9), 962-968.
- Scerri, A., Innes, A., & Scerri, C. (2017). Dementia training programmes for staff working in general hospital settings–a systematic review of the literature. Aging & mental health, 21(8), 783-796.
- Scerri, A., & Scerri, C. (2019). Outcomes in knowledge, attitudes and confidence of nursing staff working in nursing and residential care homes following a dementia training programme. Aging & mental health, 23(8), 919-928.
- Scerri, A., Innes, A., & Scerri, C. (2020). Person-centered dementia care in acute hospital wards—The influence of staff knowledge and attitudes. Geriatric Nursing, 41(3), 215-221.
- Scerri, A., & Schembri, P. (2022). Acute medical and surgical nurses’ knowledge and attitudes towards ageing and older persons. Educational Gerontology, 48(5), 224-236.
- Scerri C. (2021) Developing a skilled workforce: impact of a postgraduate programme on dementia knowledge, attitudes and training needs. The Journal of Prevention of Alzheimer’s Disease 8(1): 117-118. https://doi.org/10.14283/jpad.2020.52
- Saccasan N, Scerri C. (2020) Dementia knowledge, attitudes and training needs of speech-language pathology students and practitioners: a countrywide study. International Journal of Language and Communication Disorders 55(6): 955-970. https://doi.org/10.1111/1460-6984.12574
- Scerri C. (2018) Dementia: creating a knowledge-based healthcare profession. The Journal of Prevention of Alzheimer’s Disease 5(1): 85-86. http://dx.doi.org/10.14283/jpad.2017.43
- Scerri C. (2017) Knowledge of Alzheimer’s disease and training needs in final year medical and pharmacy students. Journal of Aging Research & Clinical Practice 6: 9-13. http://dx.doi.org/10.14283/jarcp.2016.122
- Zerafa N, Scerri C. (2016) Knowledge and pharmacological management of Alzheimer’s disease by managing community pharmacists: a nationwide study. International Journal of Clinical Pharmacy 38: 1416-1424. https://doi.org/10.1007/s11096-016-0380-8
- Schack Thoft, D., M. Pyer, Horsbol, A, Parkes, J.H. (2018) The Balanced Participation Model: sharing opportunities for giving people with early-stage dementia a voice in research. Dementia: the international journal of social research and practice, Dec, pp. 0-20. https://doi.org/10.1177/1471301218820208
- Ward, A., and Dobson, M. (2014) Dementia awareness training and the lessons learned. The Journal of Practice Teaching and Learning, 12(3), 25-43. DOI: 10.1921/8002120304
- Williams M & Daley S, 2020, Innovation in dementia education within undergraduate healthcare programmes: a scoping review, Nurse Education Today, https://doi.org/10.1016/j.nedt.2020.104742
- Wonnacott L, Banerjee S, Hicks B, Daley S, 2023, Understanding the experience of Time for Dementia Education Programme on Undergraduate Radiography Students, Radiography, https://doi.org/10.1016/j.radi.2023.02.020